Journal of Catalysis ( IF 7.3 ) Pub Date : 2020-08-19 , DOI: 10.1016/j.jcat.2020.08.017 Hongyang Su , Yifan Ye , Kyung-Jae Lee , Jie Zeng , Bongjin S. Mun , Ethan J. Crumlin
|
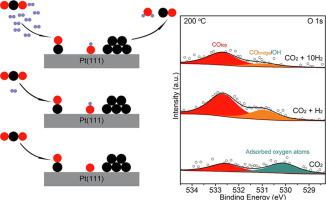
Using ambient pressure XPS (APXPS), we explored carbon dioxide (CO2) adsorption and CO2 hydrogenation on Pt(1 1 1) single crystal surface to observe the activation of CO2 and the subsequent reaction mechanism. In pure CO2, we observed CO adsorbates and adsorbed oxygen on Pt(1 1 1) derived from CO2 dissociation at room temperature. The introduction of H2 (at a pressure ratio of 1:1 (H2:CO2)) increased the production of CO across all temperatures by facilitating the removal of surface oxygen. As a consequence, the surface could expose sites that could then be utilized for producing CO. Under these conditions, the reverse water–gas shift (RWGS) reaction was observed starting at 300 oC. At higher H2 partial pressure (10:1 (H2:CO2)), the RWGS reaction initiated at a lower temperature of 200 oC and continued to enhance the conversion of CO2 with increasing temperatures. Our results revealed that CO2 was activated on a clean Pt(1 1 1) surface through the dissociation mechanism to form adsorbed CO and O at room temperature and at elevated temperatures. Introducing H2 facilitated the RWGS as adsorbed oxygen was consumed continuously to form H2O, and adsorbed CO desorbed from the surface at elevated temperatures. This work clearly provides direct experimental evidence for the surface chemistry of CO2 dissociation and demonstrates how hydrogen impacts the RWGS reaction on a platinum surface.
中文翻译:

使用环境压力X射线光电子能谱探测Pt(1 1 1)上的水煤气逆反应的表面化学
使用环境压力XPS(APXPS),我们探索了Pt(1 1 1)单晶表面上的二氧化碳(CO 2)吸附和CO 2加氢,以观察CO 2的活化及其后续反应机理。在纯CO 2中,我们观察到CO在室温下源自CO 2分解的Pt(1 1 1)上吸附并吸附了氧气。引入H 2(压力比为1:1(H 2:CO 2))通过促进表面氧的去除增加了在所有温度下CO的产生。结果,表面可能暴露出可用于产生CO的位置。在这些条件下,观察到从300 o C开始的水煤气逆反应(RWGS)。在更高的H 2分压(10:1 (H 2:CO 2)),在200更低的温度下开始的RWGS反应Ò C和继续加强CO的转化2随温度。我们的结果表明,CO 2通过离解机理在干净的Pt(1 1 1)表面上活化了三氧化二砷,在室温和升高的温度下形成吸附的一氧化碳和氧。引入H 2促进了RWGS,因为吸收的氧气不断消耗形成H 2 O,并且在高温下从表面脱附的吸收的CO。这项工作清楚地为CO 2解离的表面化学提供了直接的实验证据,并证明了氢如何影响铂表面上的RWGS反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号