当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microwave assisted synthesis, biological activities, and in silico investigation of some benzimidazole derivatives
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-08-19 , DOI: 10.1002/jhet.4129 Zeel A. Bhavsar 1 , Prachi T. Acharya 1 , Divya J. Jethava 1 , Dhaval B. Patel 1 , Mahesh S. Vasava 2 , Dhanji P. Rajani 3 , Edwin Pithawala 4 , Hitesh D. Patel 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-08-19 , DOI: 10.1002/jhet.4129 Zeel A. Bhavsar 1 , Prachi T. Acharya 1 , Divya J. Jethava 1 , Dhaval B. Patel 1 , Mahesh S. Vasava 2 , Dhanji P. Rajani 3 , Edwin Pithawala 4 , Hitesh D. Patel 1
Affiliation
|
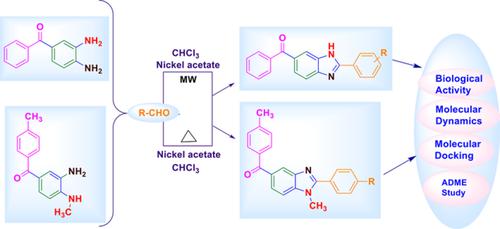
Some derivatives of 2‐substituted benzimidazole were prepared via coupling of N‐methyl‐o‐phenylenediamine or o‐phenylenediamine with different aromatic aldehydes catalyzed by Ni(OAc)2 in the presence of chloroform under microwave‐assisted conditions. Structural confirmation of all synthesized molecules was investigated by FT‐IR, 13C NMR, 1H NMR, ESI‐MS, and Elemental analysis. All prepared molecules were examined for in‐vitro pharmaceutical activities like antibacterial, antifungal, antimalarial, antituberculosis, and anti‐oxidant. Additionally, in silico study was also carried out. We also evaluated the steadiness and molecular interaction of docked complex, that is, complex of molecule (7s) with PDB: 5ZNI and complex of derivative (7l) with PDB: 3VLN, we have established a molecular dynamics model on the best dock molecules. All newly prepared molecules were authenticated to have excellent pharmacokinetics stuffs via calculated ADME‐Tox descriptors, which signifying that these derivatives could be utilized as hit for the expansion of the some innovative active compounds.
中文翻译:

微波辅助合成,生物活性和某些苯并咪唑衍生物的计算机模拟研究
在微波辅助条件下,在氯仿存在下,N-甲基-邻苯二胺或邻苯二胺与Ni(OAc)2催化的不同芳族醛偶联,制得了2-取代苯并咪唑的一些衍生物。所有合成分子的结构确认均通过FT-IR,13 C NMR,1H NMR,ESI-MS和元素分析。检查所有制备的分子的体外药物活性,例如抗菌,抗真菌,抗疟疾,抗结核和抗氧化剂。另外,还进行了计算机模拟研究。我们还评估了对接复合物(即分子(7s)与PDB:5ZNI的复合物和衍生物(7l)与PDB:3VLN的复合物)的稳定性和分子相互作用。,我们已经建立了基于最佳对接分子的分子动力学模型。通过计算的ADME-Tox描述符对所有新制备的分子进行了鉴定,使其具有出色的药代动力学物质,这表明这些衍生物可被用作扩展某些创新活性化合物的原料。
更新日期:2020-08-19
中文翻译:

微波辅助合成,生物活性和某些苯并咪唑衍生物的计算机模拟研究
在微波辅助条件下,在氯仿存在下,N-甲基-邻苯二胺或邻苯二胺与Ni(OAc)2催化的不同芳族醛偶联,制得了2-取代苯并咪唑的一些衍生物。所有合成分子的结构确认均通过FT-IR,13 C NMR,1H NMR,ESI-MS和元素分析。检查所有制备的分子的体外药物活性,例如抗菌,抗真菌,抗疟疾,抗结核和抗氧化剂。另外,还进行了计算机模拟研究。我们还评估了对接复合物(即分子(7s)与PDB:5ZNI的复合物和衍生物(7l)与PDB:3VLN的复合物)的稳定性和分子相互作用。,我们已经建立了基于最佳对接分子的分子动力学模型。通过计算的ADME-Tox描述符对所有新制备的分子进行了鉴定,使其具有出色的药代动力学物质,这表明这些衍生物可被用作扩展某些创新活性化合物的原料。


























 京公网安备 11010802027423号
京公网安备 11010802027423号