当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural insight into the effect of polymorphic variation on the functional dynamics of methionine synthase reductase: Implications in neural tube defects
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-08-19 , DOI: 10.1111/cbdd.13780 Susanta Sadhukhan 1 , Subhajit Maity 1, 2 , Sandipan Chakraborty 3 , Silpita Paul 1, 4 , Dinesh Munian 5 , Arup Kumar Pattanayak 1 , Biman Jana 6 , Madhusudan Das 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-08-19 , DOI: 10.1111/cbdd.13780 Susanta Sadhukhan 1 , Subhajit Maity 1, 2 , Sandipan Chakraborty 3 , Silpita Paul 1, 4 , Dinesh Munian 5 , Arup Kumar Pattanayak 1 , Biman Jana 6 , Madhusudan Das 1
Affiliation
|
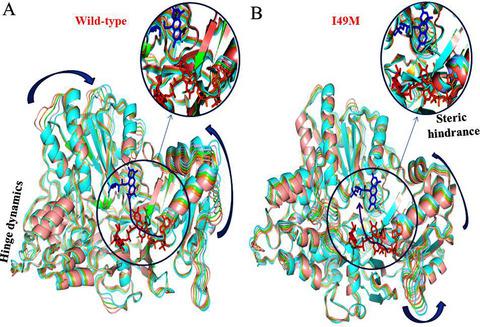
Neural tube defects (NTDs), one of the most common birth defects, are strongly associated with the variations of several single nucleotide polymorphisms (SNPs) in the MTRR gene. The gene codes a key enzyme that is involved in the rejuvenation of methionine synthase activity. An allelic variant of the protein leads to missense mutation at 49th position from isoleucine to methionine (I49M) is associated with higher disease prevalence in different populations. Here, extensive molecular dynamics simulations and interaction network analysis reveal that the 49th isoleucine is a crucial residue that allosterically regulates the dynamics between the flavin mononucleotide (FMN) and NADP(H) binding domains. I49M variation alters the functional dynamics in a way that might impede the electron transport chain along the NADP(H) → flavin adenine dinucleotide → FMN pathway. The present study provides functional insights into the effect of the genetic variations of the MTRR gene on the NTDs disease pathogenesis.
中文翻译:

多态性变异对甲硫氨酸合酶还原酶功能动力学影响的结构洞察:对神经管缺陷的影响
神经管缺陷 (NTD) 是最常见的出生缺陷之一,与MTRR中几种单核苷酸多态性 (SNP) 的变异密切相关基因。该基因编码一种关键酶,该酶参与恢复甲硫氨酸合酶活性。该蛋白质的等位基因变异导致第 49 位从异亮氨酸到甲硫氨酸 (I49M) 的错义突变与不同人群中较高的疾病患病率相关。在这里,广泛的分子动力学模拟和相互作用网络分析表明,第 49 位异亮氨酸是一种关键的残基,可通过变构调节黄素单核苷酸 (FMN) 和 NADP(H) 结合域之间的动力学。I49M 变异以可能阻碍电子传递链沿 NADP(H) → 黄素腺嘌呤二核苷酸 → FMN 途径的方式改变功能动力学。本研究提供了对MTRR遗传变异影响的功能见解 基因对 NTDs 发病机制的影响。
更新日期:2020-08-19
中文翻译:

多态性变异对甲硫氨酸合酶还原酶功能动力学影响的结构洞察:对神经管缺陷的影响
神经管缺陷 (NTD) 是最常见的出生缺陷之一,与MTRR中几种单核苷酸多态性 (SNP) 的变异密切相关基因。该基因编码一种关键酶,该酶参与恢复甲硫氨酸合酶活性。该蛋白质的等位基因变异导致第 49 位从异亮氨酸到甲硫氨酸 (I49M) 的错义突变与不同人群中较高的疾病患病率相关。在这里,广泛的分子动力学模拟和相互作用网络分析表明,第 49 位异亮氨酸是一种关键的残基,可通过变构调节黄素单核苷酸 (FMN) 和 NADP(H) 结合域之间的动力学。I49M 变异以可能阻碍电子传递链沿 NADP(H) → 黄素腺嘌呤二核苷酸 → FMN 途径的方式改变功能动力学。本研究提供了对MTRR遗传变异影响的功能见解 基因对 NTDs 发病机制的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号