Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2020-08-19 , DOI: 10.1016/j.xcrp.2020.100149 Qingyun Yan , Jinlong Zhang , Mingyang Xing
|
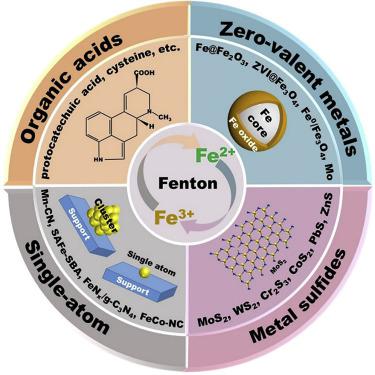
In the field of environmental science, the ideal Fenton reaction is, by definition, Fe2+-catalyzed production of hydroxyl radicals from H2O2. However, in reality, most of the reported Fenton reactions are not real catalytic reactions but redox processes, since when Fe2+ activates H2O2, it becomes Fe3+, and the return to Fe2+ can be difficult. The introduction of a cocatalyst can solve this problem. Here, we review the recent development of typical cocatalytic Fenton strategies for pollutant control, including cocatalysts based on organic acids, zero-valent metals, single atoms, and metal sulfides. The catalytic mechanisms are summarized and cocatalyst regenerations and advantages are examined. It is hoped that our review will enable chemists to develop new catalysts and cocatalysts while focusing on how to use the simplest chemical methods to make the Fenton system work in a stable manner in the long run.
中文翻译:

助催化Fenton反应控制污染物
在环境科学领域中,理想的芬顿反应,按照定义,是Fe 2+催化由H 2 O 2产生的羟基自由基。然而,实际上,大多数报道的芬顿反应不是真正的催化反应而是氧化还原过程,因为当Fe 2+激活H 2 O 2时,它变为Fe 3+,并返回到Fe 2+可能很难。助催化剂的引入可以解决这个问题。在这里,我们回顾了用于污染物控制的典型共催化Fenton策略的最新进展,这些策略包括基于有机酸,零价金属,单原子和金属硫化物的助催化剂。总结了催化机理,并考察了助催化剂的再生和优点。希望我们的审查将使化学家能够开发新的催化剂和助催化剂,同时侧重于如何使用最简单的化学方法使Fenton系统长期稳定运行。


























 京公网安备 11010802027423号
京公网安备 11010802027423号