当前位置:
X-MOL 学术
›
J. Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing the interaction between human serum albumin and the sodium dodecyl sulphate with fluorescence correlation spectroscopy
Journal of Chemical Sciences ( IF 1.7 ) Pub Date : 2020-08-18 , DOI: 10.1007/s12039-020-01816-y Vaishali Samant , Arghya Dey , G Naresh Patwari
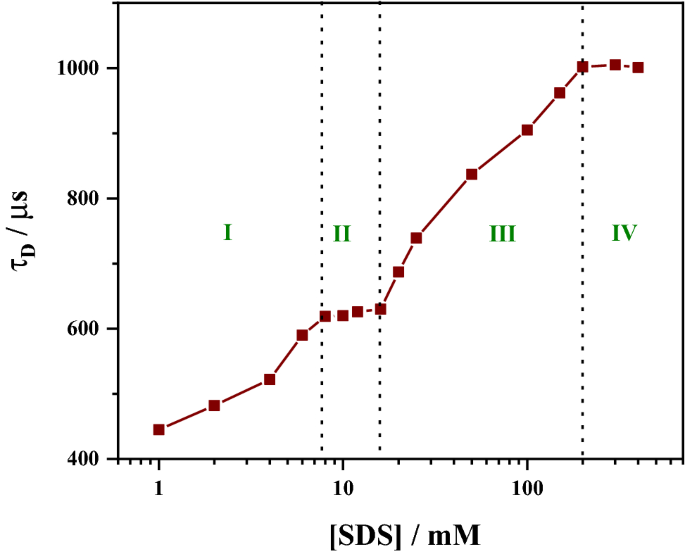 Fluorescence correlation spectroscopy reveals a four-step interaction regime between SDS and HSA. The initial opening of the tertiary structure, followed by non-specific aggregation and opening up the secondary structure and finally leading to formation of protein bound micelles with an effective increase of hydrodynamic radius by a factor of 2.6.
Fluorescence correlation spectroscopy reveals a four-step interaction regime between SDS and HSA. The initial opening of the tertiary structure, followed by non-specific aggregation and opening up the secondary structure and finally leading to formation of protein bound micelles with an effective increase of hydrodynamic radius by a factor of 2.6.
中文翻译:

用荧光相关光谱法研究人血清白蛋白与十二烷基硫酸钠之间的相互作用
 荧光相关光谱揭示了SDS和HSA之间的四步相互作用机制。最初打开三级结构,然后进行非特异性聚集并打开二级结构,最后导致形成结合蛋白的胶束,其流体动力学半径有效增加了2.6倍。
荧光相关光谱揭示了SDS和HSA之间的四步相互作用机制。最初打开三级结构,然后进行非特异性聚集并打开二级结构,最后导致形成结合蛋白的胶束,其流体动力学半径有效增加了2.6倍。
Journal of Chemical Sciences ( IF 1.7 ) Pub Date : 2020-08-18 , DOI: 10.1007/s12039-020-01816-y Vaishali Samant , Arghya Dey , G Naresh Patwari
|
|
Abstract
The denaturation of human serum albumin (HSA) upon interaction with the surfactant sodium dodecyl sulphate (SDS) was examined by measuring the diffusion time of fluorophore (RITC) tagged HSA under near single-molecule conditions using fluorescence correlation spectroscopy. The diffusion time shows four distinct regions as a function of SDS concentration, which corresponds to (I) opening of the tertiary structure, (II) non-specific SDS aggregation, (III) opening of the secondary structure, and (IV) aggregation of SDS around the secondary structure. Diffusion time increases from 383 µs for the free protein to 1002 µs for the SDS bound protein, which leads to an effective increase in the hydrodynamic radius by a factor of about 2.6.Graphic abstract
 Fluorescence correlation spectroscopy reveals a four-step interaction regime between SDS and HSA. The initial opening of the tertiary structure, followed by non-specific aggregation and opening up the secondary structure and finally leading to formation of protein bound micelles with an effective increase of hydrodynamic radius by a factor of 2.6.
Fluorescence correlation spectroscopy reveals a four-step interaction regime between SDS and HSA. The initial opening of the tertiary structure, followed by non-specific aggregation and opening up the secondary structure and finally leading to formation of protein bound micelles with an effective increase of hydrodynamic radius by a factor of 2.6.中文翻译:

用荧光相关光谱法研究人血清白蛋白与十二烷基硫酸钠之间的相互作用
摘要
通过使用荧光相关光谱法在接近单分子的条件下测量荧光团(RITC)标记的HSA的扩散时间来检查与表面活性剂十二烷基硫酸钠(SDS)相互作用时人血清白蛋白(HSA)的变性。扩散时间显示出四个不同的区域随SDS浓度的变化,分别对应于(I)三级结构的开放,(II)非特异性SDS聚集,(III)二级结构的开放和(IV)SDS浓度的聚集。 SDS围绕二级结构。扩散时间从游离蛋白的383 µs增加到SDS结合蛋白的1002 µs,这导致流体动力学半径有效增加了约2.6倍。图形摘要
 荧光相关光谱揭示了SDS和HSA之间的四步相互作用机制。最初打开三级结构,然后进行非特异性聚集并打开二级结构,最后导致形成结合蛋白的胶束,其流体动力学半径有效增加了2.6倍。
荧光相关光谱揭示了SDS和HSA之间的四步相互作用机制。最初打开三级结构,然后进行非特异性聚集并打开二级结构,最后导致形成结合蛋白的胶束,其流体动力学半径有效增加了2.6倍。


























 京公网安备 11010802027423号
京公网安备 11010802027423号