当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Customizing a Tridomain TRAIL Variant to Achieve Active Tumor Homing and Endogenous Albumin-Controlled Release of the Molecular Machine In Vivo.
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-08-17 , DOI: 10.1021/acs.biomac.0c00785 Ze Tao 1 , Yuehua Liu 1 , Hao Yang 1 , Yanru Feng 1 , Heng Li 1 , Qiuxiao Shi 1 , Shengfu Li 1 , Jingqiu Cheng 1, 2 , Xiaofeng Lu 1, 2
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-08-17 , DOI: 10.1021/acs.biomac.0c00785 Ze Tao 1 , Yuehua Liu 1 , Hao Yang 1 , Yanru Feng 1 , Heng Li 1 , Qiuxiao Shi 1 , Shengfu Li 1 , Jingqiu Cheng 1, 2 , Xiaofeng Lu 1, 2
Affiliation
|
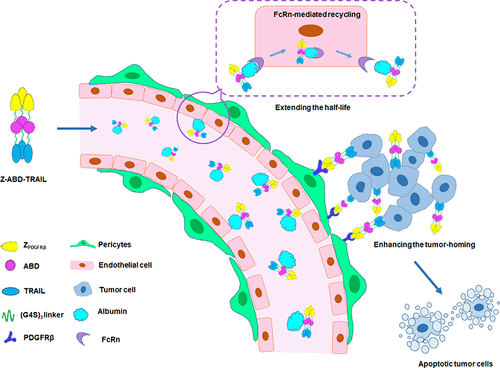
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an attractive antitumor drug candidate for precision cancer therapy due to its superior selective cytotoxicity in a variety of tumor cells. However, the clinical application of TRAIL in cancer therapy has been limited by its poor tumor-homing capacities and short half-life. Herein, we designed a tridomain TRAIL variant, Z-ABD-TRAIL, by sequentially fusing the platelet-derived growth factor receptor beta (PDGFRβ)-specific affibody ZPDGFRβ and an albumin-binding domain (ABD) to the N-terminus of TRAIL. The fusion protein Z-ABD-TRAIL was produced as a soluble protein with high yield in Escherichia coli (E. coli). The ZPDGFRβ domain provided Z-ABD-TRAIL with PDGFRβ-binding properties and thus promoted its tumor homing via the engagement of PDGFRβ-expressing pericytes on tumor microvessels. ABD-mediated binding of Z-ABD-TRAIL to albumin in the blood endowed TRAIL with long-lasting (>72 h for Z-ABD-TRAIL vs <0.5 h for TRAIL) abilities to kill tumor cells. Although the in vitro cytotoxicity of Z-ABD-TRAIL in tumor cells was similar to that of the parent TRAIL, the in vivo tumor uptake, apoptosis-inducing ability, and antitumor effect of Z-ABD-TRAIL were much greater than those of TRAIL, indicating that ZPDGFRβ-mediated tumor homing and ABD-introduced albumin binding significantly improved the pharmacodynamics of TRAIL. In addition, repeated injection of high-dose Z-ABD-TRAIL showed no obvious acute toxicity in mice. These results demonstrate that the newly designed tridomain Z-ABD-TRAIL is a promising agent for precision cancer therapy.
中文翻译:

定制Tridomain TRAIL变体以实现主动肿瘤归巢和分子机器体内内源性白蛋白控制释放。
肿瘤坏死因子相关的凋亡诱导配体(TRAIL)是精密癌症治疗的一种有吸引力的抗肿瘤药物候选物,因为它在多种肿瘤细胞中具有优越的选择性细胞毒性。但是,TRAIL在癌症治疗中的临床应用受到其肿瘤归巢能力差和半衰期短的限制。在这里,我们通过将血小板衍生的生长因子受体β(PDGFRβ)特异性亲和体ZPDGFRβ和白蛋白结合域(ABD)依次融合到TRAIL的N端,设计了三域TRAIL变体Z-ABD- TRAIL 。融合蛋白Z-ABD-TRAIL在大肠杆菌(E.coli)中以高产量产生为可溶性蛋白。在Z PDGFRβ结构域提供具有PDGFRβ结合特性的Z-ABD-TRAIL,并因此通过表达PDGFRβ的周细胞在肿瘤微血管上的接合而促进其肿瘤归巢。ABD介导的Z-ABD-TRAIL与血液中白蛋白的结合赋予TRAIL杀死肿瘤细胞的持久能力(Z-ABD-TRAIL> 72小时,TRAIL <0.5小时)。尽管Z-ABD-TRAIL在肿瘤细胞中的体外细胞毒性与亲本TRAIL相似,但是Z-ABD-TRAIL的体内肿瘤吸收,凋亡诱导能力和抗肿瘤作用远大于TRAIL。 ,表示ZPDGFRβ介导的肿瘤归巢和ABD引入的白蛋白结合显着改善了TRAIL的药效学。此外,重复注射大剂量Z-ABD-TRAIL对小鼠没有明显的急性毒性。这些结果表明,新设计的三结构域Z-ABD-TRAIL是用于精确癌症治疗的有前途的药物。
更新日期:2020-10-12
中文翻译:

定制Tridomain TRAIL变体以实现主动肿瘤归巢和分子机器体内内源性白蛋白控制释放。
肿瘤坏死因子相关的凋亡诱导配体(TRAIL)是精密癌症治疗的一种有吸引力的抗肿瘤药物候选物,因为它在多种肿瘤细胞中具有优越的选择性细胞毒性。但是,TRAIL在癌症治疗中的临床应用受到其肿瘤归巢能力差和半衰期短的限制。在这里,我们通过将血小板衍生的生长因子受体β(PDGFRβ)特异性亲和体ZPDGFRβ和白蛋白结合域(ABD)依次融合到TRAIL的N端,设计了三域TRAIL变体Z-ABD- TRAIL 。融合蛋白Z-ABD-TRAIL在大肠杆菌(E.coli)中以高产量产生为可溶性蛋白。在Z PDGFRβ结构域提供具有PDGFRβ结合特性的Z-ABD-TRAIL,并因此通过表达PDGFRβ的周细胞在肿瘤微血管上的接合而促进其肿瘤归巢。ABD介导的Z-ABD-TRAIL与血液中白蛋白的结合赋予TRAIL杀死肿瘤细胞的持久能力(Z-ABD-TRAIL> 72小时,TRAIL <0.5小时)。尽管Z-ABD-TRAIL在肿瘤细胞中的体外细胞毒性与亲本TRAIL相似,但是Z-ABD-TRAIL的体内肿瘤吸收,凋亡诱导能力和抗肿瘤作用远大于TRAIL。 ,表示ZPDGFRβ介导的肿瘤归巢和ABD引入的白蛋白结合显着改善了TRAIL的药效学。此外,重复注射大剂量Z-ABD-TRAIL对小鼠没有明显的急性毒性。这些结果表明,新设计的三结构域Z-ABD-TRAIL是用于精确癌症治疗的有前途的药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号