当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A novel function of Mycobacterium tuberculosis chaperonin paralog GroEL1 in copper homeostasis
FEBS Letters ( IF 3.5 ) Pub Date : 2020-09-16 , DOI: 10.1002/1873-3468.13906 Mohammed Yousuf Ansari 1 , Sakshi D Batra 2 , Hina Ojha 1 , Kanika Dhiman 3 , Ashish Ganguly 3 , Jaya S Tyagi 2 , Shekhar C Mande 1
FEBS Letters ( IF 3.5 ) Pub Date : 2020-09-16 , DOI: 10.1002/1873-3468.13906 Mohammed Yousuf Ansari 1 , Sakshi D Batra 2 , Hina Ojha 1 , Kanika Dhiman 3 , Ashish Ganguly 3 , Jaya S Tyagi 2 , Shekhar C Mande 1
Affiliation
|
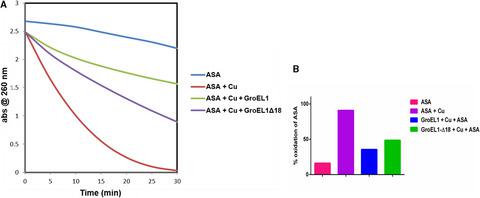
Among the two GroEL paralogs in Mycobacterium tuberculosis, GroEL1 and GroEL2, GroEL1 has a characteristic histidine‐rich C terminus. Since histidine richness is likely to be involved in metal binding, we attempted to decipher the role of GroEL1 in chelating metals and the consequence on M. tuberculosis physiology. Isothermal titration calorimetry showed that GroEL1 binds copper and other metals. Mycobacterial viability assay, redox balance, and DNA protection assay concluded that GroEL1 protects from copper stress in vitro. Solution X‐ray scattering and constrained modeling of GroEL1 −/+ copper ions showed reorientation of the apical domain as seen in functional assembly. We conclude that the duplication of chaperonin genes in M. tuberculosis might have led to their evolutionary divergence and consequent functional divergence of chaperonins.
中文翻译:

结核分枝杆菌伴侣蛋白旁系同源物 GroEL1 在铜稳态中的新功能
在结核分枝杆菌的两个 GroEL 旁系同源物 GroEL1 和 GroEL2 中,GroEL1 具有特征性的富含组氨酸的 C 末端。由于组氨酸丰富可能与金属结合有关,我们试图破译 GroEL1 在螯合金属中的作用以及对结核分枝杆菌生理的影响。等温滴定量热法显示 GroEL1 结合铜和其他金属。分枝杆菌活力测定、氧化还原平衡和 DNA 保护测定得出结论,GroEL1 可保护体外铜应激。GroEL1 -/+ 铜离子的溶液 X 射线散射和约束建模显示了顶端域的重新定向,如功能组装中所见。我们得出结论,结核分枝杆菌中伴侣蛋白基因的重复可能导致它们的进化分歧和随之而来的伴侣蛋白功能分歧。
更新日期:2020-09-16
中文翻译:

结核分枝杆菌伴侣蛋白旁系同源物 GroEL1 在铜稳态中的新功能
在结核分枝杆菌的两个 GroEL 旁系同源物 GroEL1 和 GroEL2 中,GroEL1 具有特征性的富含组氨酸的 C 末端。由于组氨酸丰富可能与金属结合有关,我们试图破译 GroEL1 在螯合金属中的作用以及对结核分枝杆菌生理的影响。等温滴定量热法显示 GroEL1 结合铜和其他金属。分枝杆菌活力测定、氧化还原平衡和 DNA 保护测定得出结论,GroEL1 可保护体外铜应激。GroEL1 -/+ 铜离子的溶液 X 射线散射和约束建模显示了顶端域的重新定向,如功能组装中所见。我们得出结论,结核分枝杆菌中伴侣蛋白基因的重复可能导致它们的进化分歧和随之而来的伴侣蛋白功能分歧。


























 京公网安备 11010802027423号
京公网安备 11010802027423号