当前位置:
X-MOL 学术
›
Miner. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorption of ferric ions on the surface of bastnaesite and its significance in flotation
Minerals Engineering ( IF 4.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.mineng.2020.106588 Xinyu Zhu , Yiming Lin , Yang Huang , Yangge Zhu , Changxing Shi , Weiqing Wang
Minerals Engineering ( IF 4.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.mineng.2020.106588 Xinyu Zhu , Yiming Lin , Yang Huang , Yangge Zhu , Changxing Shi , Weiqing Wang
|
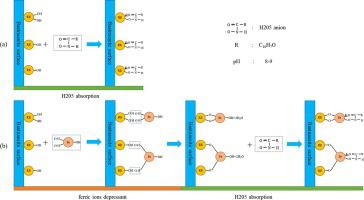
Abstract In the system of 3-hydroxy-2-naphthyl hydroxamic acid (H205) collector, the adsorption of ferric ions on the surface of bastnaesite and its significance in flotation was investigated by micro-flotation tests, zeta potential measurements, adsorption experiments, solution chemistry analysis, X-ray photoelectron spectroscopy (XPS) measurements and density functional theory (DFT) calculations. From the results of our experiments, it can be known that ferric ions species (Fe(OH)3(s)) can absorb on the surface of bastnaesite by interacting with RE(OH)x3−x (x = 1, 2) (RE = Ce, La and Nd) to form RE-O-Fe-OH. And in this process, Fe(OH)3(s) is bound to RE(OH)x3−x (x = 1, 2) mainly by bidentate adsorption. By bidentate adsorption with RE(OH)x3−x (x = 1, 2), the addition of ferric ions decreases adsorption sites for H205, impedes the adsorption of H205 on the surface of bastnaesite, and then depresses bastnaesite flotation.
中文翻译:

三价铁离子在氟碳铈矿表面的吸附及其浮选意义
摘要 在 3-羟基-2-萘基异羟肟酸 (H2O5) 捕收剂体系中,通过微浮选试验、zeta 电位测量、吸附实验、溶液法研究了三价铁离子在氟碳铈矿表面的吸附及其在浮选中的意义。化学分析、X 射线光电子能谱 (XPS) 测量和密度泛函理论 (DFT) 计算。从我们的实验结果可以知道,铁离子物质(Fe(OH)3(s))可以通过与RE(OH)x3−x(x = 1, 2)相互作用而吸附在氟碳铈矿表面上( RE = Ce、La 和 Nd) 形成 RE-O-Fe-OH。在这个过程中,Fe(OH)3(s)主要通过双齿吸附与RE(OH)x3−x (x = 1, 2)结合。通过与 RE(OH)x3−x (x = 1, 2) 的双齿吸附,铁离子的添加减少了 H2O5 的吸附位点,
更新日期:2020-11-01
中文翻译:

三价铁离子在氟碳铈矿表面的吸附及其浮选意义
摘要 在 3-羟基-2-萘基异羟肟酸 (H2O5) 捕收剂体系中,通过微浮选试验、zeta 电位测量、吸附实验、溶液法研究了三价铁离子在氟碳铈矿表面的吸附及其在浮选中的意义。化学分析、X 射线光电子能谱 (XPS) 测量和密度泛函理论 (DFT) 计算。从我们的实验结果可以知道,铁离子物质(Fe(OH)3(s))可以通过与RE(OH)x3−x(x = 1, 2)相互作用而吸附在氟碳铈矿表面上( RE = Ce、La 和 Nd) 形成 RE-O-Fe-OH。在这个过程中,Fe(OH)3(s)主要通过双齿吸附与RE(OH)x3−x (x = 1, 2)结合。通过与 RE(OH)x3−x (x = 1, 2) 的双齿吸附,铁离子的添加减少了 H2O5 的吸附位点,


























 京公网安备 11010802027423号
京公网安备 11010802027423号