当前位置:
X-MOL 学术
›
Lett. Appl. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preventing contamination of PCR‐based multiplex assays including the use of a dedicated biosafety cabinet
Letters in Applied Microbiology ( IF 2.4 ) Pub Date : 2020-10-18 , DOI: 10.1111/lam.13375 A. Bouam 1, 2 , J.J. Vincent 3 , M. Drancourt 2, 3 , D. Raoult 2, 3 , P.Y. Levy 1, 3
Letters in Applied Microbiology ( IF 2.4 ) Pub Date : 2020-10-18 , DOI: 10.1111/lam.13375 A. Bouam 1, 2 , J.J. Vincent 3 , M. Drancourt 2, 3 , D. Raoult 2, 3 , P.Y. Levy 1, 3
Affiliation
|
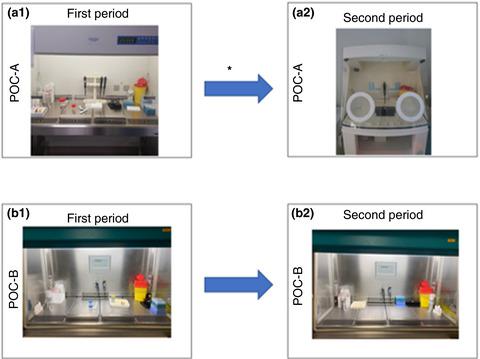
We retrospectively investigated cases of false‐positive diagnoses using the BIOFIRE® FilmArray® meningitis/encephalitis (ME) panel to measure the impact of using a dedicated biosafety cabinet combined with preventive measures to reduce the prevalence of false‐positive diagnoses due to pre‐analytical in‐laboratory contamination. False‐positive results were identified by reviewing clinical data, biological parameters and cytology results of cerebrospinal fluid (CSF) samples showing discrepant results between the FilmArray ME panel and routine PCR assays. A total of 327 CSF were analysed over 16 weeks in point‐of‐care (POC) A and B, over two 8‐week periods, periods 1 and 2. The analysis yielded 30 (9·17%) detection of at least one pathogen including 21/30 (70%) viruses and 9/30 (30%) bacteria. During period 1, POC‐A and POC‐B manipulated CSF under a non‐dedicated hood featuring laminar flow, whereas during period 2, CSFs were manipulated under a dedicated biosafety cabinet without any airflow in POC‐A. During period 1, false positives were detected in 3/114 CSF (2·63%) in POC‐A and 1/36 (2·77%) in POC‐B (P = 0·97); during period 2, false positives were detected in 0/139 CSF (0%) in POC‐A and 1/38 (2·63%) in POC‐B (P = 0·23). All false positives were bacterial. The use of a dedicated cabinet without ventilation along with preventive measures during period 2 in POC‐A significantly reduced the number of false‐positive results (P = 0·05). Preventive measures described in this study can mitigate false positives when using PCR‐based multiplex assays such as the BIOFIRE FilmArray ME Panel for the diagnosis of meningitis and other infectious diseases.
中文翻译:

防止基于 PCR 的多重检测受到污染,包括使用专用的生物安全柜
我们使用 BIOFIRE® FilmArray® 脑膜炎/脑炎 (ME) 面板对假阳性诊断案例进行了回顾性调查,以衡量使用专用生物安全柜结合预防措施的影响,以减少由于预分析导致的假阳性诊断的发生率。实验室内污染。假阳性结果是通过审查脑脊液 (CSF) 样本的临床数据、生物学参数和细胞学结果来确定的,这些结果显示 FilmArray ME 面板和常规 PCR 检测之间存在差异。在护理点 (POC) A 和 B 的 16 周内,在两个 8 周期间,期间 1 和 2 中分析了总共 327 例 CSF。分析产生了 30 (9·17%) 次检测到至少一种病原体包括 21/30 (70%) 病毒和 9/30 (30%) 细菌。在第 1 期,POC-A 和 POC-B 在具有层流特征的非专用罩子下操纵脑脊液,而在第 2 阶段,脑脊液在专用生物安全柜下操纵,POC-A 中没有任何气流。在第 1 阶段,POC-A 中 3/114 CSF (2·63%) 和 POC-B 中 1/36 (2·77%) 检测到假阳性(P = 0·97);在第 2 阶段,POC-A 中 0/139 CSF (0%) 和 POC-B 中 1/38 (2·63%) 检测到假阳性(P = 0·23)。所有假阳性都是细菌。在 POC-A 的第 2 阶段使用不通风的专用柜以及预防措施显着减少了假阳性结果的数量(P = 0·05)。当使用基于 PCR 的多重检测(例如 BIOFIRE FilmArray ME Panel)诊断脑膜炎和其他传染病时,本研究中描述的预防措施可以减少假阳性。
更新日期:2020-10-18
中文翻译:

防止基于 PCR 的多重检测受到污染,包括使用专用的生物安全柜
我们使用 BIOFIRE® FilmArray® 脑膜炎/脑炎 (ME) 面板对假阳性诊断案例进行了回顾性调查,以衡量使用专用生物安全柜结合预防措施的影响,以减少由于预分析导致的假阳性诊断的发生率。实验室内污染。假阳性结果是通过审查脑脊液 (CSF) 样本的临床数据、生物学参数和细胞学结果来确定的,这些结果显示 FilmArray ME 面板和常规 PCR 检测之间存在差异。在护理点 (POC) A 和 B 的 16 周内,在两个 8 周期间,期间 1 和 2 中分析了总共 327 例 CSF。分析产生了 30 (9·17%) 次检测到至少一种病原体包括 21/30 (70%) 病毒和 9/30 (30%) 细菌。在第 1 期,POC-A 和 POC-B 在具有层流特征的非专用罩子下操纵脑脊液,而在第 2 阶段,脑脊液在专用生物安全柜下操纵,POC-A 中没有任何气流。在第 1 阶段,POC-A 中 3/114 CSF (2·63%) 和 POC-B 中 1/36 (2·77%) 检测到假阳性(P = 0·97);在第 2 阶段,POC-A 中 0/139 CSF (0%) 和 POC-B 中 1/38 (2·63%) 检测到假阳性(P = 0·23)。所有假阳性都是细菌。在 POC-A 的第 2 阶段使用不通风的专用柜以及预防措施显着减少了假阳性结果的数量(P = 0·05)。当使用基于 PCR 的多重检测(例如 BIOFIRE FilmArray ME Panel)诊断脑膜炎和其他传染病时,本研究中描述的预防措施可以减少假阳性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号