当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient synthesis of α-amino secondary amides by direct aminocarbonylation of N-Boc-imines using carbamoylsilane as an amide source
Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-15 , DOI: 10.1016/j.tet.2020.131476 Qilin Guo , Minggang Zhao , Jianxin Chen
中文翻译:

使用氨基甲酰基硅烷作为酰胺源,通过N-Boc-亚胺的直接氨基羰基化反应,高效合成α-氨基仲酰胺
更新日期:2020-08-28
Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-15 , DOI: 10.1016/j.tet.2020.131476 Qilin Guo , Minggang Zhao , Jianxin Chen
|
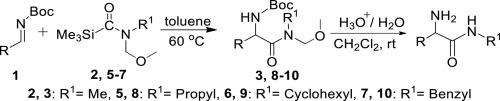
A novel and practical procedure for the preparation of α-amino secondary amides by the aminocarbonylation of N-Boc-imines using carbamoylsilanes bearing methoxymethyl group as secondary amides source is presented herein. The protocol tolerates N-Boc-imines bearing different groups, including aryl, double bond conjugated aryl, heteroaryl and aliphatic groups. The reactions provide good to excellent yields of the products under mild and catalysts-free conditions. The electronic property and the steric hindrance of substituent on the N-Boc-imines affect the reaction.
中文翻译:

使用氨基甲酰基硅烷作为酰胺源,通过N-Boc-亚胺的直接氨基羰基化反应,高效合成α-氨基仲酰胺
本文提出了一种新颖且实用的方法,该方法通过使用带有甲氧基甲基的氨基甲酰基硅烷作为仲酰胺源,通过N-Boc-亚胺的氨基羰基化来制备α-氨基仲酰胺。该方案容许带有不同基团的N-Boc-亚胺,包括芳基,双键共轭芳基,杂芳基和脂族基团。在温和且无催化剂的条件下,反应可提供良好的产品收率。N-Boc-亚胺上取代基的电子性质和位阻会影响反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号