当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Silver Adsorption on Calcium Niobate(001) Nanosheets: Calorimetric Energies Explain Sinter-Resistant Support
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-08-14 , DOI: 10.1021/jacs.0c05044 Wei Zhang 1 , Ritesh Uppuluri 2 , Thomas E Mallouk 2, 3, 4 , Charles T Campbell 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-08-14 , DOI: 10.1021/jacs.0c05044 Wei Zhang 1 , Ritesh Uppuluri 2 , Thomas E Mallouk 2, 3, 4 , Charles T Campbell 1
Affiliation
|
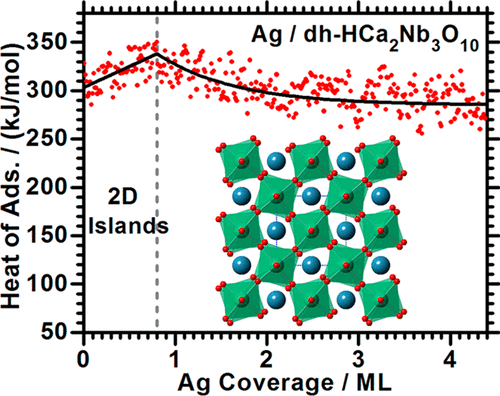
Metal nanoparticles deposited on oxide supports are essential to many technologies, including catalysts, fuel cells and electronics. Therefore, understanding the chemical bonding strength between metal nanoparticles and oxide surfaces is of great interest. The adsorption energetics, adhesion energy and adsorbate structure of Ag on dehydrated HCa2Nb3O10(001) nanosheets at 300 K have been studied using metal adsorption calorimetry and surface spectroscopies. These dehydrated ("dh") calcium niobate nanosheets "(dh-HCa2Nb3O10(001))" have stoichiometry Ca4Nb6O19. They impart unusual stability to metal nanoparticles when used as catalyst supports and are easy-to-prepare by Langmuir-Blodgett (LB) techniques, highly ordered, and essentially single-crystal surfaces of mixed oxides with a huge ratio of terrace to edge sites. Below monolayer coverage, Ag grows on dh-HCa2Nb3O10(001) as 2D islands of thickness ~2 layers. The differential heat of Ag adsorption is initially ~303 kJ/mol, increasing slowly to ~338 kJ/mol by 0.8 ML. At higher coverages, Ag atoms mainly add on top of these 2D islands, growing 3D nanoparticles of increasing thickness, as the heat decreases asymptotically towards silver's heat of sublimation (285 kJ/mol). The adhesion energy of Ag(s) to this Ca niobate surface is estimated to be 4.33 J/m2, larger than on any oxide surface previously measured. This explains the sinter resistance reported for metal nanoparticles on this support. Electron transfer from Ag into the calcium niobate is also measured. These results demonstrate an easy way to do single-crystal-type surface science studies - and especially thermochemical measurements - on the complex surfaces of mixed oxides: using LB-deposited perovskite nanosheets and ultrahigh vacuum annealing in O2.
中文翻译:

铌酸钙 (001) 纳米片上的银吸附:量热能解释耐烧结支撑
沉积在氧化物载体上的金属纳米粒子对许多技术至关重要,包括催化剂、燃料电池和电子产品。因此,了解金属纳米粒子与氧化物表面之间的化学键合强度具有重要意义。使用金属吸附量热法和表面光谱法研究了 300 K 下 Ag 在脱水 HCa2Nb3O10(001) 纳米片上的吸附能量、粘附能和吸附结构。这些脱水(“dh”)铌酸钙纳米片“(dh-HCa2Nb3O10(001))”具有化学计量Ca4Nb6O19。当用作催化剂载体时,它们赋予金属纳米粒子异常的稳定性,并且易于通过 Langmuir-Blodgett (LB) 技术制备,具有高度有序的混合氧化物的基本单晶表面,具有巨大的阶地与边缘位点的比例。在单层覆盖以下,Ag 在 dh-HCa2Nb3O10(001) 上生长为厚度约 2 层的二维岛。Ag 吸附的差热最初为 ~303 kJ/mol,通过 0.8 ML 缓慢增加到 ~338 kJ/mol。在更高的覆盖率下,Ag 原子主要添加到这些 2D 岛的顶部,随着热量向银的升华热 (285 kJ/mol) 逐渐降低,从而生长出厚度增加的 3D 纳米粒子。Ag(s) 对该铌酸钙表面的粘附能估计为 4.33 J/m2,大于先前测量的任何氧化物表面。这解释了该载体上金属纳米颗粒的耐烧结性。还测量了从 Ag 到铌酸钙的电子转移。
更新日期:2020-08-14
中文翻译:

铌酸钙 (001) 纳米片上的银吸附:量热能解释耐烧结支撑
沉积在氧化物载体上的金属纳米粒子对许多技术至关重要,包括催化剂、燃料电池和电子产品。因此,了解金属纳米粒子与氧化物表面之间的化学键合强度具有重要意义。使用金属吸附量热法和表面光谱法研究了 300 K 下 Ag 在脱水 HCa2Nb3O10(001) 纳米片上的吸附能量、粘附能和吸附结构。这些脱水(“dh”)铌酸钙纳米片“(dh-HCa2Nb3O10(001))”具有化学计量Ca4Nb6O19。当用作催化剂载体时,它们赋予金属纳米粒子异常的稳定性,并且易于通过 Langmuir-Blodgett (LB) 技术制备,具有高度有序的混合氧化物的基本单晶表面,具有巨大的阶地与边缘位点的比例。在单层覆盖以下,Ag 在 dh-HCa2Nb3O10(001) 上生长为厚度约 2 层的二维岛。Ag 吸附的差热最初为 ~303 kJ/mol,通过 0.8 ML 缓慢增加到 ~338 kJ/mol。在更高的覆盖率下,Ag 原子主要添加到这些 2D 岛的顶部,随着热量向银的升华热 (285 kJ/mol) 逐渐降低,从而生长出厚度增加的 3D 纳米粒子。Ag(s) 对该铌酸钙表面的粘附能估计为 4.33 J/m2,大于先前测量的任何氧化物表面。这解释了该载体上金属纳米颗粒的耐烧结性。还测量了从 Ag 到铌酸钙的电子转移。


























 京公网安备 11010802027423号
京公网安备 11010802027423号