当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New Twists in Nazarov Cyclization Chemistry.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-08-13 , DOI: 10.1021/acs.accounts.0c00284 Alison J Frontier 1 , Jackson J Hernandez 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-08-13 , DOI: 10.1021/acs.accounts.0c00284 Alison J Frontier 1 , Jackson J Hernandez 1
Affiliation
|
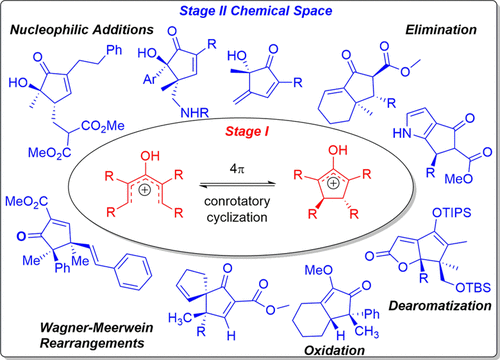
The defining feature of the Nazarov cyclization is a 4π-conrotatory electrocyclization, resulting in the stereospecific formation of functionalized cyclopentanones. The reaction provides access to structural motifs that are found in many natural products and drug targets. Harnessing the full potential of the Nazarov cyclization broadens its utility by enabling the development of new methodologies and synthetic strategies. To achieve these goals through efficient cyclization design, it is helpful to think of the reaction as a two-stage process. The first stage involves a 4π-electrocyclization leading to the formation of an allylic cation, and the second stage corresponds to the fate of this cationic intermediate. With a complete understanding of the discrete events that characterize the overall process, one can optimize reactivity and control the selectivity of the different Stage 2 pathways.
中文翻译:

纳扎罗夫环化化学的新进展。
纳扎罗夫环化反应的主要特征是4π旋转电环化反应,导致功能化环戊酮的立体定向形成。该反应提供了进入许多天然产物和药物靶标中发现的结构基序的途径。利用Nazarov环化的全部潜力,可以通过开发新的方法和综合策略来扩大其用途。为了通过有效的环化设计实现这些目标,将反应视为两个阶段的过程将很有帮助。第一阶段涉及4π电环化,导致形成烯丙基阳离子,第二阶段对应于该阳离子中间体的命运。全面了解表征整个过程的离散事件后,
更新日期:2020-09-15
中文翻译:

纳扎罗夫环化化学的新进展。
纳扎罗夫环化反应的主要特征是4π旋转电环化反应,导致功能化环戊酮的立体定向形成。该反应提供了进入许多天然产物和药物靶标中发现的结构基序的途径。利用Nazarov环化的全部潜力,可以通过开发新的方法和综合策略来扩大其用途。为了通过有效的环化设计实现这些目标,将反应视为两个阶段的过程将很有帮助。第一阶段涉及4π电环化,导致形成烯丙基阳离子,第二阶段对应于该阳离子中间体的命运。全面了解表征整个过程的离散事件后,



























 京公网安备 11010802027423号
京公网安备 11010802027423号