Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tubulin Double Helix: Lateral and Longitudinal Curvature Changes of Tubulin Protofilament.
Small ( IF 13.3 ) Pub Date : 2020-08-13 , DOI: 10.1002/smll.202001240 Juncheol Lee 1 , Chaeyeon Song 1 , Jimin Lee 1 , Herbert P Miller 2 , Hasaeam Cho 1 , Bopil Gim 1 , Youli Li 3 , Stuart C Feinstein 2 , Leslie Wilson 2 , Cyrus R Safinya 4 , Myung Chul Choi 1
Small ( IF 13.3 ) Pub Date : 2020-08-13 , DOI: 10.1002/smll.202001240 Juncheol Lee 1 , Chaeyeon Song 1 , Jimin Lee 1 , Herbert P Miller 2 , Hasaeam Cho 1 , Bopil Gim 1 , Youli Li 3 , Stuart C Feinstein 2 , Leslie Wilson 2 , Cyrus R Safinya 4 , Myung Chul Choi 1
Affiliation
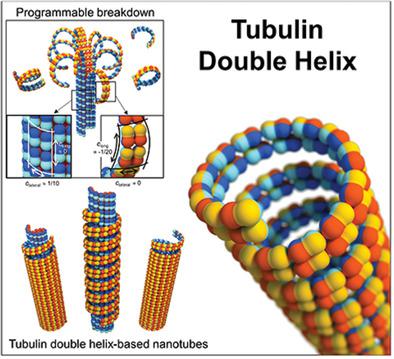
|
By virtue of their native structures, tubulin dimers are protein building blocks that are naturally preprogrammed to assemble into microtubules (MTs), which are cytoskeletal polymers. Here, polycation‐directed (i.e., electrostatically tunable) assembly of tubulins is demonstrated by conformational changes to the tubulin protofilament in longitudinal and lateral directions, creating tubulin double helices and various tubular architectures. Synchrotron small‐angle X‐ray scattering and transmission electron microscopy reveal a remarkable range of nanoscale assembly structures: single‐ and double‐layered double‐helix tubulin tubules. The phase transitions from MTs to the new assemblies are dependent on the size and concentration of polycations. Two characteristic scales that determine the number of observed phases are the size of polycation compared to the size of tubulin (≈4 nm) and to MT diameter (≈25 nm). This work suggests the feasibility of using polycations that have scissor‐ and glue‐like properties to achieve “programmable breakdown” of protein nanotubes, tearing MTs into double‐stranded tubulins and building up previously undiscovered nanostructures. Importantly, a new role of tubulins is defined as 2D shape‐controllable building blocks for supramolecular architectures. These findings provide insight into the design of protein‐based functional materials, for example, as metallization templates for nanoscale electronic devices, molecular screws, and drug delivery vehicles.
中文翻译:

微管蛋白双螺旋:微管蛋白原丝的横向和纵向曲率变化。
凭借其天然结构,微管蛋白二聚体是蛋白质构件,可以自然地预先编程为组装成微管(MTs),它们是细胞骨架聚合物。在这里,通过微管蛋白原丝在纵向和横向上的构象变化,证明了微管蛋白的聚阳离子导向(即静电可调)组装,产生了微管蛋白双螺旋和各种管状结构。同步加速器小角度X射线散射和透射电子显微镜揭示了一系列纳米级组装结构:单层和双层双螺旋微管蛋白管。从MT到新组件的相变取决于聚阳离子的大小和浓度。决定观察到的相数的两个特征量表是聚阳离子的大小与微管蛋白的大小(≈4nm)和MT直径(≈25nm)相比。这项工作表明,使用具有剪刀和胶状特性的聚阳离子来实现蛋白质纳米管的“可编程分解”,将MT撕成双链微管蛋白并建立以前未发现的纳米结构是可行的。重要的是,微管蛋白的新作用被定义为超分子体系结构的2D形状可控构建基块。这些发现为基于蛋白质的功能材料的设计提供了见识,例如,作为纳米级电子设备,分子螺钉和药物输送工具的金属化模板。这项工作表明,使用具有剪刀和胶状特性的聚阳离子来实现蛋白质纳米管的“可编程分解”,将MT撕成双链微管蛋白并建立以前未发现的纳米结构是可行的。重要的是,微管蛋白的新作用被定义为超分子体系结构的2D形状可控构建基块。这些发现为基于蛋白质的功能材料的设计提供了见识,例如,作为纳米级电子设备,分子螺钉和药物输送工具的金属化模板。这项工作表明,使用具有剪刀和胶状特性的聚阳离子来实现蛋白质纳米管的“可编程分解”,将MT撕成双链微管蛋白并建立以前未发现的纳米结构是可行的。重要的是,微管蛋白的新作用被定义为超分子体系结构的2D形状可控构建基块。这些发现为基于蛋白质的功能材料的设计提供了见识,例如,作为纳米级电子设备,分子螺钉和药物输送工具的金属化模板。微管蛋白的新作用被定义为超分子体系结构的2D形状可控构建基块。这些发现为基于蛋白质的功能材料的设计提供了见识,例如,作为纳米级电子设备,分子螺钉和药物输送工具的金属化模板。微管蛋白的新作用被定义为超分子体系结构的2D形状可控构建基块。这些发现为基于蛋白质的功能材料的设计提供了见识,例如,作为纳米级电子设备,分子螺钉和药物输送工具的金属化模板。
更新日期:2020-09-18
中文翻译:

微管蛋白双螺旋:微管蛋白原丝的横向和纵向曲率变化。
凭借其天然结构,微管蛋白二聚体是蛋白质构件,可以自然地预先编程为组装成微管(MTs),它们是细胞骨架聚合物。在这里,通过微管蛋白原丝在纵向和横向上的构象变化,证明了微管蛋白的聚阳离子导向(即静电可调)组装,产生了微管蛋白双螺旋和各种管状结构。同步加速器小角度X射线散射和透射电子显微镜揭示了一系列纳米级组装结构:单层和双层双螺旋微管蛋白管。从MT到新组件的相变取决于聚阳离子的大小和浓度。决定观察到的相数的两个特征量表是聚阳离子的大小与微管蛋白的大小(≈4nm)和MT直径(≈25nm)相比。这项工作表明,使用具有剪刀和胶状特性的聚阳离子来实现蛋白质纳米管的“可编程分解”,将MT撕成双链微管蛋白并建立以前未发现的纳米结构是可行的。重要的是,微管蛋白的新作用被定义为超分子体系结构的2D形状可控构建基块。这些发现为基于蛋白质的功能材料的设计提供了见识,例如,作为纳米级电子设备,分子螺钉和药物输送工具的金属化模板。这项工作表明,使用具有剪刀和胶状特性的聚阳离子来实现蛋白质纳米管的“可编程分解”,将MT撕成双链微管蛋白并建立以前未发现的纳米结构是可行的。重要的是,微管蛋白的新作用被定义为超分子体系结构的2D形状可控构建基块。这些发现为基于蛋白质的功能材料的设计提供了见识,例如,作为纳米级电子设备,分子螺钉和药物输送工具的金属化模板。这项工作表明,使用具有剪刀和胶状特性的聚阳离子来实现蛋白质纳米管的“可编程分解”,将MT撕成双链微管蛋白并建立以前未发现的纳米结构是可行的。重要的是,微管蛋白的新作用被定义为超分子体系结构的2D形状可控构建基块。这些发现为基于蛋白质的功能材料的设计提供了见识,例如,作为纳米级电子设备,分子螺钉和药物输送工具的金属化模板。微管蛋白的新作用被定义为超分子体系结构的2D形状可控构建基块。这些发现为基于蛋白质的功能材料的设计提供了见识,例如,作为纳米级电子设备,分子螺钉和药物输送工具的金属化模板。微管蛋白的新作用被定义为超分子体系结构的2D形状可控构建基块。这些发现为基于蛋白质的功能材料的设计提供了见识,例如,作为纳米级电子设备,分子螺钉和药物输送工具的金属化模板。



























 京公网安备 11010802027423号
京公网安备 11010802027423号