JACC: Heart Failure ( IF 13.0 ) Pub Date : 2020-08-12 , DOI: 10.1016/j.jchf.2020.06.008 David D Berg 1 , Eugene Braunwald 1 , Adam D DeVore 2 , Anuradha Lala 3 , Sean P Pinney 3 , Carol I Duffy 4 , Yared Gurmu 1 , Eric J Velazquez 5 , David A Morrow 1
|
Objectives
This study sought to evaluate the efficacy and safety of sacubitril/valsartan according to dose level achieved in the PIONEER-HF (Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode) trial.
Background
In patients hospitalized for acute decompensated heart failure (ADHF), in-hospital initiation and continuation of sacubitril/valsartan as compared with enalapril is well tolerated, achieves a greater reduction in N-terminal pro–B-type natriuretic peptide (NT-proBNP), and reduces the risk of cardiovascular death or rehospitalization for HF through 8 weeks. However, not all patients achieve the target dose of sacubitril/valsartan, and its efficacy and safety in such patients are of interest.
Methods
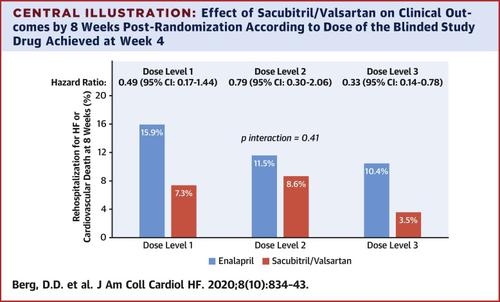
PIONEER-HF was a randomized, double-blind, active-controlled trial of sacubitril/valsartan versus enalapril in 881 patients stabilized during hospitalization for ADHF. Blinded study medication was administered for 8 weeks, with initial dosing selected based on the systolic blood pressure at randomization and titrated toward a target of sacubitril/valsartan 97/103 mg twice daily, or enalapril 10 mg twice daily, with an algorithm based on systolic blood pressure and the investigator’s assessment of tolerability.
Results
At 4 weeks, 199 (55%) patients allocated to sacubitril/valsartan and 211 (60%) patients allocated to enalapril were dispensed the target dose. Baseline characteristics were similar in the 2 treatment groups within each dose level. There was no heterogeneity across dose levels in the effect of sacubitril/valsartan on the reduction in NT-proBNP (pinteraction = 0.69), the reduction in cardiovascular death or rehospitalization for heart failure (pinteraction = 0.42), or the pre-specified adverse events of special interest through 8 weeks.
Conclusions
In hemodynamically stabilized patients with ADHF, the efficacy and safety of sacubitril/valsartan are generally consistent across dose levels. (Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode [PIONEER-HF]; NCT02554890)
中文翻译:

PIONEER-HF 试验中达到的剂量水平的沙库巴曲/缬沙坦的疗效和安全性。
目标
本研究旨在根据 PIONEER-HF(沙库巴曲/缬沙坦与依那普利对急性心力衰竭发作稳定的患者中 NT-proBNP 影响的比较)试验中达到的剂量水平评估沙库巴曲/缬沙坦的疗效和安全性。
背景
在因急性失代偿性心力衰竭 (ADHF) 住院的患者中,与依那普利相比,院内开始和继续使用沙库巴曲/缬沙坦具有良好的耐受性,可实现 N 端 B 型利钠肽原 (NT-proBNP) 更大程度的降低,并通过 8 周降低心血管死亡或 HF 再住院的风险。然而,并非所有患者都能达到沙库巴曲/缬沙坦的目标剂量,其在此类患者中的有效性和安全性值得关注。
方法
PIONEER-HF 是一项在 881 名因 ADHF 住院期间病情稳定的患者中进行的沙库巴曲/缬沙坦与依那普利的随机、双盲、活性对照试验。盲法研究药物给药 8 周,根据随机分组时的收缩压选择初始剂量,并朝着目标沙库巴曲/缬沙坦 97/103 mg 每天两次或依那普利 10 mg 每天两次,使用基于收缩压的算法血压和研究者对耐受性的评估。
结果
在第 4 周时,分配给沙库巴曲/缬沙坦的 199 名患者(55%)和分配给依那普利的 211 名(60%)患者分配了目标剂量。在每个剂量水平内,2 个治疗组的基线特征相似。沙库巴曲/缬沙坦对 NT-proBNP 降低(p交互作用 = 0.69)、心血管死亡或心力衰竭再住院减少(p交互作用 = 0.42)或预先指定的影响在剂量水平之间没有异质性8 周内特别关注的不良事件。
结论
在血液动力学稳定的 ADHF 患者中,沙库巴曲/缬沙坦的疗效和安全性在不同剂量水平上通常是一致的。(沙库巴曲/缬沙坦与依那普利对急性心力衰竭稳定患者 NT-proBNP 影响的比较 [PIONEER-HF];NCT02554890)


























 京公网安备 11010802027423号
京公网安备 11010802027423号