当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biomaterials as Local Niches for Immunomodulation.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-08-13 , DOI: 10.1021/acs.accounts.0c00341 Kwasi Adu-Berchie 1, 2 , David J Mooney 1, 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-08-13 , DOI: 10.1021/acs.accounts.0c00341 Kwasi Adu-Berchie 1, 2 , David J Mooney 1, 2
Affiliation
|
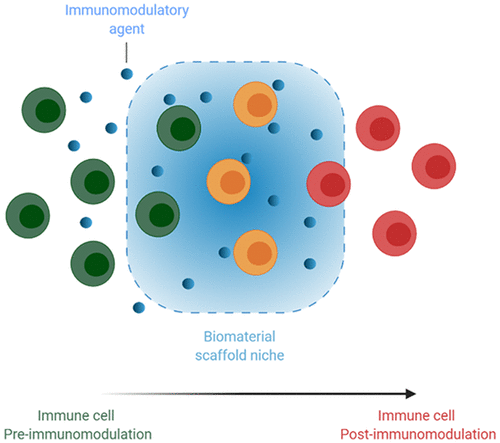
A major function of the immune system is to detect threat from foreign invaders, tissue damage, or cancer and to mount a counter response that resolves the threat, restores homeostasis, and supplies immunological memory to prevent a second assault. Our increasing understanding of the immune system has opened up numerous avenues for modulating immune responses against infections, cancer, and autoimmunity. However, agents used for immunomodulation have been traditionally administered systemically via bolus injection, leading to unintended consequences by disrupting homeostasis at nontarget sites. Consequently, systemic hyperactivation and hypoactivation can result from bolus administration of immune-activators and immunosuppressants, respectively. Macroscale biomaterial scaffolds can instead be placed at the intended target site to provide both localized, controlled release of immunomodulatory agents and control over local immune cell trafficking and function, potentially maximizing therapeutic efficacy and limiting systemic exposure. These scaffolds have found utility in the area of cancer immunotherapy, especially in situ cancer vaccination where controlled release of factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and the local presentation of tumor antigen and danger signals lead to the recruitment of immature dendritic cells and facilitate their activation and antigen presentation. These cells eventually migrate into secondary lymphoid organs where they prime tumor specific T cells for downstream tumor clearance. Scaffolds can also be used in adoptive T cell therapy to generate large numbers of potent antigen specific T cells or chimeric antigen receptor (CAR) T cells in vitro for subsequent delivery to patients. Macroscale biomaterial scaffolds have also found utility beyond cancer immunotherapy and have been developed to promote immune tolerance by regulatory T cell induction and to expedite tissue regeneration. The design of these macroscale biomaterial scaffolds considers their biocompatibility, biodegradability, mode of delivery, porosity, and kinetics of therapeutic cargo release. Consequently, the numerous approaches that have been developed to fabricate biomaterial scaffolds are aimed at tuning these parameters to achieve the desired therapeutic outcome. This Account will discuss the use of biomaterial scaffolds as niches for immunomodulation and will focus on (1) approaches that have been used to fabricate various biomaterial systems being employed as niches for immunomodulation and (2) how these biomaterial systems have been used to modulate immune responses, specifically in area of cancer immunotherapy, where we will discuss the role of macroscale biomaterial scaffolds for in situ vaccination and in vitro T cell expansion. We will also briefly discuss the utility of biomaterial scaffolds beyond cancer, drawing examples from tolerance and tissue regeneration.
中文翻译:

生物材料作为免疫调节的局部壁ches。
免疫系统的主要功能是检测来自外来入侵者,组织损伤或癌症的威胁,并提出应对措施,以解决威胁,恢复体内平衡并提供免疫记忆以防止第二次攻击。我们对免疫系统的日益了解为调节针对感染,癌症和自身免疫的免疫反应开辟了许多途径。然而,用于免疫调节的试剂传统上已通过推注注射全身给药,通过破坏非靶位点的稳态而导致意想不到的后果。因此,分别推注免疫激活剂和免疫抑制剂可导致全身性过度激活和过度激活。相反,可以将大型生物材料支架放置在预期的靶位点上,以提供免疫调节剂的局部控制释放以及对局部免疫细胞运输和功能的控制,从而可能最大化治疗功效并限制全身性暴露。这些支架已发现可用于癌症免疫治疗领域,尤其是原位癌症疫苗接种,其中如粒细胞-巨噬细胞集落刺激因子(GM-CSF)的控制释放以及肿瘤抗原和危险信号的局部表达导致未成熟树突状细胞的募集并促进其活化和抗原呈递。这些细胞最终迁移到次级淋巴器官中,在那里它们引发肿瘤特异性T细胞以清除下游肿瘤。支架也可用于过继性T细胞疗法中,以在体外产生大量有效的抗原特异性T细胞或嵌合抗原受体(CAR)T细胞,以随后递送给患者。大型生物材料支架还发现了癌症免疫疗法以外的用途,并已开发出通过调节性T细胞诱导来促进免疫耐受并加速组织再生的方法。这些大型生物材料支架的设计考虑了它们的生物相容性,生物降解性,递送方式,孔隙率以及治疗性货物释放的动力学。因此,已开发出的用于制造生物材料支架的众多方法旨在调节这些参数以实现所需的治疗结果。该帐户将讨论如何使用生物材料支架作为免疫调节的壁and,并将着重于(1)已用于制造用作免疫调节的壁垒的各种生物材料系统的方法,以及(2)这些生物材料系统如何用于调节免疫力反应,特别是在癌症免疫治疗领域,我们将在此讨论大规模生物材料支架在治疗中的作用。生物降解性,递送方式,孔隙率和治疗性货物释放的动力学。因此,已开发出的用于制造生物材料支架的众多方法旨在调节这些参数以实现所需的治疗结果。该帐户将讨论如何使用生物材料支架作为免疫调节的壁and,并将着重于(1)已用于制造用作免疫调节的壁垒的各种生物材料系统的方法,以及(2)这些生物材料系统如何用于调节免疫力反应,特别是在癌症免疫治疗领域,我们将在此讨论大规模生物材料支架在治疗中的作用。生物降解性,递送方式,孔隙率和治疗性货物释放的动力学。因此,已开发出的用于制造生物材料支架的众多方法旨在调节这些参数以实现所需的治疗结果。该帐户将讨论如何使用生物材料支架作为免疫调节的壁and,并将着重于(1)已用于制造用作免疫调节的壁垒的各种生物材料系统的方法,以及(2)这些生物材料系统如何用于调节免疫力反应,特别是在癌症免疫治疗领域,我们将在此讨论大规模生物材料支架在治疗中的作用。已经开发出许多制造生物材料支架的方法,旨在调节这些参数以实现所需的治疗结果。该帐户将讨论如何使用生物材料支架作为免疫调节的壁and,并将着重于(1)已用于制造用作免疫调节的壁垒的各种生物材料系统的方法,以及(2)这些生物材料系统如何用于调节免疫力反应,特别是在癌症免疫治疗领域,我们将在此讨论大规模生物材料支架在治疗中的作用。已经开发出许多制造生物材料支架的方法,旨在调节这些参数以实现所需的治疗结果。该帐户将讨论如何使用生物材料支架作为免疫调节的壁and,并将着重于(1)已用于制造用作免疫调节的壁垒的各种生物材料系统的方法,以及(2)这些生物材料系统如何用于调节免疫力反应,特别是在癌症免疫治疗领域,我们将在此讨论大规模生物材料支架在治疗中的作用。原位疫苗接种和体外T细胞扩增。我们还将简要介绍生物材料支架在癌症以外的用途,并从耐受性和组织再生中汲取实例。
更新日期:2020-09-15
中文翻译:

生物材料作为免疫调节的局部壁ches。
免疫系统的主要功能是检测来自外来入侵者,组织损伤或癌症的威胁,并提出应对措施,以解决威胁,恢复体内平衡并提供免疫记忆以防止第二次攻击。我们对免疫系统的日益了解为调节针对感染,癌症和自身免疫的免疫反应开辟了许多途径。然而,用于免疫调节的试剂传统上已通过推注注射全身给药,通过破坏非靶位点的稳态而导致意想不到的后果。因此,分别推注免疫激活剂和免疫抑制剂可导致全身性过度激活和过度激活。相反,可以将大型生物材料支架放置在预期的靶位点上,以提供免疫调节剂的局部控制释放以及对局部免疫细胞运输和功能的控制,从而可能最大化治疗功效并限制全身性暴露。这些支架已发现可用于癌症免疫治疗领域,尤其是原位癌症疫苗接种,其中如粒细胞-巨噬细胞集落刺激因子(GM-CSF)的控制释放以及肿瘤抗原和危险信号的局部表达导致未成熟树突状细胞的募集并促进其活化和抗原呈递。这些细胞最终迁移到次级淋巴器官中,在那里它们引发肿瘤特异性T细胞以清除下游肿瘤。支架也可用于过继性T细胞疗法中,以在体外产生大量有效的抗原特异性T细胞或嵌合抗原受体(CAR)T细胞,以随后递送给患者。大型生物材料支架还发现了癌症免疫疗法以外的用途,并已开发出通过调节性T细胞诱导来促进免疫耐受并加速组织再生的方法。这些大型生物材料支架的设计考虑了它们的生物相容性,生物降解性,递送方式,孔隙率以及治疗性货物释放的动力学。因此,已开发出的用于制造生物材料支架的众多方法旨在调节这些参数以实现所需的治疗结果。该帐户将讨论如何使用生物材料支架作为免疫调节的壁and,并将着重于(1)已用于制造用作免疫调节的壁垒的各种生物材料系统的方法,以及(2)这些生物材料系统如何用于调节免疫力反应,特别是在癌症免疫治疗领域,我们将在此讨论大规模生物材料支架在治疗中的作用。生物降解性,递送方式,孔隙率和治疗性货物释放的动力学。因此,已开发出的用于制造生物材料支架的众多方法旨在调节这些参数以实现所需的治疗结果。该帐户将讨论如何使用生物材料支架作为免疫调节的壁and,并将着重于(1)已用于制造用作免疫调节的壁垒的各种生物材料系统的方法,以及(2)这些生物材料系统如何用于调节免疫力反应,特别是在癌症免疫治疗领域,我们将在此讨论大规模生物材料支架在治疗中的作用。生物降解性,递送方式,孔隙率和治疗性货物释放的动力学。因此,已开发出的用于制造生物材料支架的众多方法旨在调节这些参数以实现所需的治疗结果。该帐户将讨论如何使用生物材料支架作为免疫调节的壁and,并将着重于(1)已用于制造用作免疫调节的壁垒的各种生物材料系统的方法,以及(2)这些生物材料系统如何用于调节免疫力反应,特别是在癌症免疫治疗领域,我们将在此讨论大规模生物材料支架在治疗中的作用。已经开发出许多制造生物材料支架的方法,旨在调节这些参数以实现所需的治疗结果。该帐户将讨论如何使用生物材料支架作为免疫调节的壁and,并将着重于(1)已用于制造用作免疫调节的壁垒的各种生物材料系统的方法,以及(2)这些生物材料系统如何用于调节免疫力反应,特别是在癌症免疫治疗领域,我们将在此讨论大规模生物材料支架在治疗中的作用。已经开发出许多制造生物材料支架的方法,旨在调节这些参数以实现所需的治疗结果。该帐户将讨论如何使用生物材料支架作为免疫调节的壁and,并将着重于(1)已用于制造用作免疫调节的壁垒的各种生物材料系统的方法,以及(2)这些生物材料系统如何用于调节免疫力反应,特别是在癌症免疫治疗领域,我们将在此讨论大规模生物材料支架在治疗中的作用。原位疫苗接种和体外T细胞扩增。我们还将简要介绍生物材料支架在癌症以外的用途,并从耐受性和组织再生中汲取实例。


























 京公网安备 11010802027423号
京公网安备 11010802027423号