Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective synthesis of cyclic and linear diamines by imine cycloadditions.
Chirality ( IF 2 ) Pub Date : 2020-07-03 , DOI: 10.1002/chir.23265 Tsung-Che Chang 1 , Ambara R Pradipta 1, 2 , Katsunori Tanaka 1, 2, 3, 4
Chirality ( IF 2 ) Pub Date : 2020-07-03 , DOI: 10.1002/chir.23265 Tsung-Che Chang 1 , Ambara R Pradipta 1, 2 , Katsunori Tanaka 1, 2, 3, 4
Affiliation
|
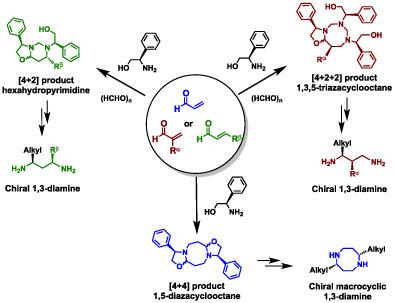
Imine is one of the most versatile functional groups in chemistry and biochemistry fields. Although many biochemical reactions involve imine formation, the inherently unstable property of N‐alkyl‐α,β‐unsaturated imines still hindered their utilization in organic synthesis. In this article, we described that the N‐alkyl‐α,β‐unsaturated imines, which prepared from alkylamines and acrolein, could smoothly react through [4 + 4] cycloaddition to give eight‐membered diazacyclooctane derivatives in excellent yields. Under a similar condition, in the presence of formaldehyde, the [4 + 2] and [4 + 2 + 2] cycloadditions could lead to the formation of six‐membered hexahydropyrimidine or eight‐membered triazacyclooctanes, depending on the substituent of aldehydes. Moreover, an easy functional group manipulation of the cyclic products obtained from these cycloadditions can provide variously substituted chiral linear diamines. We can utilize these novel reactivities to reveal the unknown and essential properties of many biological processes that involve N‐alkyl‐unsaturated imines.
中文翻译:

通过亚胺环加成反应对环和线性二胺的对映选择性合成。
亚胺是化学和生物化学领域中最通用的官能团之一。尽管许多生化反应都涉及亚胺的形成,但是N-烷基-α,β-不饱和亚胺的内在不稳定特性仍然阻碍了它们在有机合成中的利用。在本文中,我们描述了N由烷基胺和丙烯醛制备的烷基-α,β-不饱和亚胺可以通过[4 + 4]环加成反应平稳反应,从而以优异的收率得到八元二氮杂环辛烷衍生物。在相似的条件下,在甲醛存在下,根据醛的取代基,[4 + 2]和[4 + 2 + 2]环加成可导致六元六氢嘧啶或八元三氮杂环辛烷的形成。而且,由这些环加成反应得到的环状产物的容易的官能团操纵可提供各种取代的手性线性二胺。我们可以利用这些新颖的反应性来揭示涉及N烷基不饱和亚胺的许多生物过程的未知和基本特性。
更新日期:2020-07-03
中文翻译:

通过亚胺环加成反应对环和线性二胺的对映选择性合成。
亚胺是化学和生物化学领域中最通用的官能团之一。尽管许多生化反应都涉及亚胺的形成,但是N-烷基-α,β-不饱和亚胺的内在不稳定特性仍然阻碍了它们在有机合成中的利用。在本文中,我们描述了N由烷基胺和丙烯醛制备的烷基-α,β-不饱和亚胺可以通过[4 + 4]环加成反应平稳反应,从而以优异的收率得到八元二氮杂环辛烷衍生物。在相似的条件下,在甲醛存在下,根据醛的取代基,[4 + 2]和[4 + 2 + 2]环加成可导致六元六氢嘧啶或八元三氮杂环辛烷的形成。而且,由这些环加成反应得到的环状产物的容易的官能团操纵可提供各种取代的手性线性二胺。我们可以利用这些新颖的反应性来揭示涉及N烷基不饱和亚胺的许多生物过程的未知和基本特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号