Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral bioanalytical methods in bioequivalence studies of intravenous vs. oral formulations of ibuprofen.
Chirality ( IF 2 ) Pub Date : 2020-06-29 , DOI: 10.1002/chir.23258 Esperanza González-Rojano 1 , Julio Marcotegui 2 , Leonor Laredo 1, 3 , Luther Gwaza 4 , John Gordon 5 , Antonio Portolés 1, 3 , Emilio Vargas 1, 3 , Susana Morales-Alcelay 6 , Alfredo García-Arieta 6
Chirality ( IF 2 ) Pub Date : 2020-06-29 , DOI: 10.1002/chir.23258 Esperanza González-Rojano 1 , Julio Marcotegui 2 , Leonor Laredo 1, 3 , Luther Gwaza 4 , John Gordon 5 , Antonio Portolés 1, 3 , Emilio Vargas 1, 3 , Susana Morales-Alcelay 6 , Alfredo García-Arieta 6
Affiliation
|
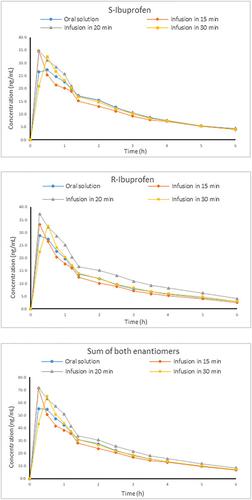
According to the Ibuprofen Product‐Specific Bioequivalence Guidance of the European Medicines Agency, achiral bioanalytical methods are considered acceptable for demonstration of bioequivalence of ibuprofen‐containing products.
中文翻译:

布洛芬静脉内和口服制剂生物等效性研究中的手性生物分析方法。
根据欧洲药品管理局的布洛芬特定产品生物等效性指南,非手性生物分析方法被认为可以证明含布洛芬产品的生物等效性。
更新日期:2020-06-29
中文翻译:

布洛芬静脉内和口服制剂生物等效性研究中的手性生物分析方法。
根据欧洲药品管理局的布洛芬特定产品生物等效性指南,非手性生物分析方法被认为可以证明含布洛芬产品的生物等效性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号