Current Organic Synthesis ( IF 1.8 ) Pub Date : 2020-11-30 , DOI: 10.2174/1570179417666200713181959 Manvinder Kaur 1, 2 , Mohamad Yusuf 2 , Dharambeer Singh Malhi 1 , Harvinder Singh Sohal 1
|
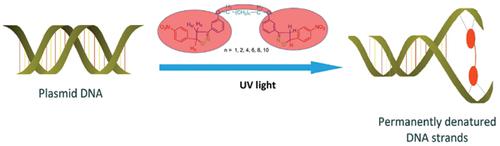
Aim and objective: Isoxazole is an active core found in many drugs. The aim of this work was to synthesize bis-isoxazoline compounds and to analyze the effect of linker chain length on biological activities.
Material and Methods: A simple, convenient, and efficient method for the conversion of bischalcones to new bis(4,5-dihydroisoxazole) derivatives was developed by using hydroxylamine hydrochloride under basic medium. Synthesized moieties were also evaluated for their antimicrobial potencies and DNA photocleavage assay.
Results and Discussion: The synthesized compounds were more active than their chalcone precursors and the long-chain linkers (4e&4f) were more potent in antimicrobial, as well as in DNA photocleavage activity.
Conclusion: It was found that many of the tested bischalcones and bis-isoxazolines exhibited moderate to significant antimicrobial activity against various strains. Furthermore, the present study also provides significant information and interesting outcomes regarding cyclization, increasing the length of linker chains, and their effects on the DNA photocleavage and antimicrobial activities.
中文翻译:

双二氢异恶唑啉:合成、结构解析、抗菌评估和 DNA 光裂解测定。
目的和目的:异恶唑是许多药物中发现的活性核心。这项工作的目的是合成双异恶唑啉化合物并分析接头链长对生物活性的影响。
材料与方法:开发了一种在碱性介质下使用盐酸羟胺将双查尔酮转化为新的双(4,5-二氢异恶唑)衍生物的简单、方便、有效的方法。还评估了合成部分的抗菌效力和 DNA 光裂解测定。
结果与讨论:合成的化合物比其查耳酮前体更具活性,长链接头 (4e&4f) 在抗菌和 DNA 光裂解活性方面更有效。
结论:发现许多测试的双查耳酮和双异恶唑啉对各种菌株表现出中等至显着的抗菌活性。此外,本研究还提供了关于环化、增加接头链长度及其对 DNA 光裂解和抗菌活性的影响的重要信息和有趣的结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号