当前位置:
X-MOL 学术
›
Biopolymers
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gelatin grafted poly( D,L ‐ lactide ) as an inhibitor of protein aggregation: An in vitro case study
Biopolymers ( IF 2.9 ) Pub Date : 2020-06-30 , DOI: 10.1002/bip.23383 Chelladurai Karthikeyan Balavigneswaran 1, 2 , Gaurav Kumar 3, 4 , Chandrasekaran Vignesh Kumar 5 , Satheeshkumar Sellamuthu 6 , Uvanesh Kasiviswanathan 7 , Biswajit Ray 8 , Vignesh Muthuvijayan 2 , Sanjeev Kumar Mahto 9 , Nira Misra 1
Biopolymers ( IF 2.9 ) Pub Date : 2020-06-30 , DOI: 10.1002/bip.23383 Chelladurai Karthikeyan Balavigneswaran 1, 2 , Gaurav Kumar 3, 4 , Chandrasekaran Vignesh Kumar 5 , Satheeshkumar Sellamuthu 6 , Uvanesh Kasiviswanathan 7 , Biswajit Ray 8 , Vignesh Muthuvijayan 2 , Sanjeev Kumar Mahto 9 , Nira Misra 1
Affiliation
|
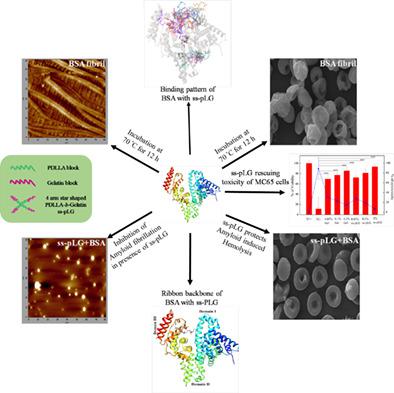
Amyloids are a group of proteins that are capable of forming aggregated amyloid fibrils, which is responsible for many neurodegenerative diseases including Alzheimer's disease (AD). In our previous study, synthesis and characterization of star‐shaped poly(D,L‐lactide)‐b‐gelatin (ss‐pLG) have been reported. In the present work, we have extended our work to study ss‐pLG against protein aggregation. To the best of our knowledge, this is the first report on the inhibition of amyloid fibrillation by protein grafted poly(D,L‐lactide). Bovine serum albumin (BSA) was chosen as the model protein, which readily forms fibril under high temperature. We found that ss‐pLG efficiently suppressed the fibril formation of BSA compared with gelatin (Gel), which was supported by Thioflavin T assay, circular dichroism (CD) spectroscopy and atomic force microscopy (AFM). In addition, ss‐pLG significantly curtailed amyloid‐induced hemolysis. We also found that incubation of ss‐pLG with neuroblastoma cells (MC65) protected the cells from fibril‐induced toxicity. The rescuing efficiency of ss‐pLG was better than Gel, which could be attributed to the reduced lamella thickness in branched ss‐pLG. These results suggest the significance of gelatin grafting, which probably allows gelatin to interact with the key residues of the amyloidogenic core of BSA effectively.
中文翻译:

明胶接枝聚(D,L-丙交酯)作为蛋白质聚集抑制剂:体外案例研究
淀粉样蛋白是一组能够形成聚集的淀粉样蛋白原纤维的蛋白质,它是导致许多神经退行性疾病,包括阿尔茨海默病 (AD) 的原因。在我们之前的研究中,已经报道了星形聚(D,L-丙交酯)-b-明胶(ss-pLG)的合成和表征。在目前的工作中,我们将我们的工作扩展到研究 ss-pLG 对蛋白质聚集的影响。据我们所知,这是第一份关于通过蛋白质接枝聚(D,L-丙交酯)抑制淀粉样蛋白纤维化的报告。选择牛血清白蛋白(BSA)作为模型蛋白,其在高温下容易形成原纤维。我们发现与明胶 (Gel) 相比,ss-pLG 有效地抑制了 BSA 的原纤维形成,这得到了硫磺素 T 测定的支持,圆二色性 (CD) 光谱和原子力显微镜 (AFM)。此外,ss-pLG 显着减少了淀粉样蛋白诱导的溶血。我们还发现 ss-pLG 与神经母细胞瘤细胞 (MC65) 的孵育保护细胞免受原纤维诱导的毒性。ss-pLG 的拯救效率优于 Gel,这可能是由于分支 ss-pLG 中薄片厚度的减少。这些结果表明明胶接枝的重要性,这可能使明胶与 BSA 淀粉样蛋白核心的关键残基有效相互作用。这可能归因于分支 ss-pLG 中薄片厚度的降低。这些结果表明明胶接枝的重要性,这可能使明胶与 BSA 淀粉样蛋白核心的关键残基有效相互作用。这可能归因于分支 ss-pLG 中薄片厚度的降低。这些结果表明明胶接枝的重要性,这可能使明胶与 BSA 淀粉样蛋白核心的关键残基有效相互作用。
更新日期:2020-06-30
中文翻译:

明胶接枝聚(D,L-丙交酯)作为蛋白质聚集抑制剂:体外案例研究
淀粉样蛋白是一组能够形成聚集的淀粉样蛋白原纤维的蛋白质,它是导致许多神经退行性疾病,包括阿尔茨海默病 (AD) 的原因。在我们之前的研究中,已经报道了星形聚(D,L-丙交酯)-b-明胶(ss-pLG)的合成和表征。在目前的工作中,我们将我们的工作扩展到研究 ss-pLG 对蛋白质聚集的影响。据我们所知,这是第一份关于通过蛋白质接枝聚(D,L-丙交酯)抑制淀粉样蛋白纤维化的报告。选择牛血清白蛋白(BSA)作为模型蛋白,其在高温下容易形成原纤维。我们发现与明胶 (Gel) 相比,ss-pLG 有效地抑制了 BSA 的原纤维形成,这得到了硫磺素 T 测定的支持,圆二色性 (CD) 光谱和原子力显微镜 (AFM)。此外,ss-pLG 显着减少了淀粉样蛋白诱导的溶血。我们还发现 ss-pLG 与神经母细胞瘤细胞 (MC65) 的孵育保护细胞免受原纤维诱导的毒性。ss-pLG 的拯救效率优于 Gel,这可能是由于分支 ss-pLG 中薄片厚度的减少。这些结果表明明胶接枝的重要性,这可能使明胶与 BSA 淀粉样蛋白核心的关键残基有效相互作用。这可能归因于分支 ss-pLG 中薄片厚度的降低。这些结果表明明胶接枝的重要性,这可能使明胶与 BSA 淀粉样蛋白核心的关键残基有效相互作用。这可能归因于分支 ss-pLG 中薄片厚度的降低。这些结果表明明胶接枝的重要性,这可能使明胶与 BSA 淀粉样蛋白核心的关键残基有效相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号