Current Pharmaceutical Biotechnology ( IF 2.8 ) Pub Date : 2020-10-31 , DOI: 10.2174/1389201021666200628152246 Iwuchukwu A Emmanuel 1 , Fisayo A Olotu 1 , Clement Agoni 1 , Mahmoud E S Soliman 1
|
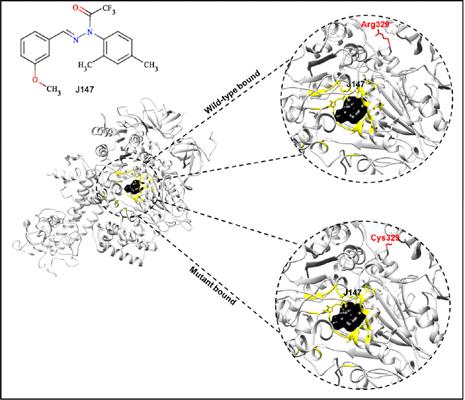
Background: Neonatal Encephalopathy (NE) is a mitochondrial ATP synthase (mATPase) disease, which results in the death of infants. The case presented here is reportedly caused by complex V deficiency as a result of mutation of Arginine to Cysteine at residue 329 in the mATPase. A recent breakthrough was the discovery of J147, which targets mATPase in the treatment of Alzheimer’s disease. Based on the concepts of computational target-based drug design, this study investigated the possibility of employing J147 as a viable candidate in the treatment of NE.
Objective/Methods: The structural dynamic implications of this drug on the mutated enzyme are yet to be elucidated. Hence, integrative molecular dynamics simulations and thermodynamic calculations were employed to investigate the activity of J147 on the mutated enzyme in comparison to its already established inhibitory activity on the wild-type enzyme.
Results: A correlated structural trend occurred between the wild-type and mutant systems whereby all the systems exhibited an overall conformational transition. Equal observations in favorable free binding energies further substantiated uniformity in the mobility, and residual fluctuation of the wild-type and mutant systems. The similarity in the binding landscape suggests that J147 could as well modulate mutant mATPase activity in addition to causing structural modifications in the wild-type enzyme.
Conclusion: Findings suggest that J147 can stabilize the mutant protein and restore it to a similar structural state as the wild-type which depicts functionality. These details could be employed in drug design for potential drug resistance cases due to mATPase mutations that may present in the future.
中文翻译:

在计算机上重用J147治疗新生儿脑病:突变线粒体ATP合酶的分子机制。
背景:新生儿脑病(NE)是一种线粒体ATP合酶(mATPase)疾病,导致婴儿死亡。据报道,此病例是由于mATPase中第329位残基的精氨酸突变为半胱氨酸而导致复杂的V缺乏引起的。最近的突破是发现J147,该J147靶向mATPase在阿尔茨海默氏病的治疗中。基于基于计算靶点的药物设计概念,本研究调查了将J147用作NE治疗的可行候选药物的可能性。
目的/方法:该药物对突变酶的结构动力学影响尚待阐明。因此,与已经确定的其对野生型酶的抑制活性相比,采用综合分子动力学模拟和热力学计算来研究J147对突变的酶的活性。
结果:在野生型和突变体系统之间发生了相关的结构趋势,由此所有系统都表现出整体构象转变。在有利的自由结合能方面的平等观察进一步证实了迁移率的均匀性,以及野生型和突变型系统的残留波动。结合态的相似性表明,J147除了在野生型酶中引起结构修饰外,还可以调节突变型mATPase活性。
结论:研究结果表明,J147可以稳定突变蛋白并将其恢复到与野生型相似的结构状态,该结构描述了功能。这些细节可能会因未来可能出现的mATPase突变而用于潜在的耐药性药物设计中。

























 京公网安备 11010802027423号
京公网安备 11010802027423号