当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Negative Allosteric Modulator of mGluR1 Improves Long-Term Neurologic Deficits after Experimental Subarachnoid Hemorrhage.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-08-11 , DOI: 10.1021/acschemneuro.0c00485 Hong-Bin Wang 1 , Wei-Qi Wang 1, 2 , Qing-Jian Wu 3 , Ya-Jun Hou 1 , Han-Xia Li 1 , Hui-Juan Yang 1 , Ming-Feng Yang 1 , Bao-Liang Sun 1 , Zong-Yong Zhang 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-08-11 , DOI: 10.1021/acschemneuro.0c00485 Hong-Bin Wang 1 , Wei-Qi Wang 1, 2 , Qing-Jian Wu 3 , Ya-Jun Hou 1 , Han-Xia Li 1 , Hui-Juan Yang 1 , Ming-Feng Yang 1 , Bao-Liang Sun 1 , Zong-Yong Zhang 1
Affiliation
|
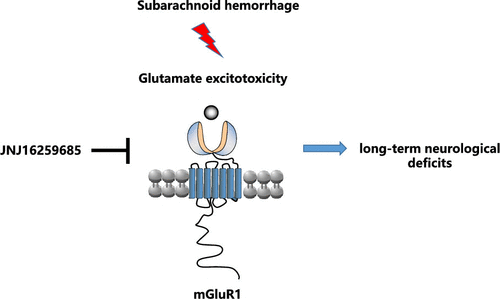
Aneurysmal subarachnoid hemorrhage (SAH) causes permanent neurological sequelae, but the underlying mechanism needs to be further clarified. Here, we show that inhibition of metabotropic glutamate receptor 1 (mGluR1) with negative allosteric modulator JNJ16259685 improves long-term neurobehavioral outcomes in an endovascular perforation model of SAH. JNJ16259685 improves cerebrovascular dysfunction through attenuation of cerebral blood flow (CBF) reduction, cerebral vasoconstrictio, and microthrombosis formation in a rat SAH model. Moreover, JNJ16259685 reduces experimental SAH-induced long-term neuronal damage through alleviation of neuronal death and degeneration. Mechanically, JNJ16259685 maintains phosphorylation of endothelial NO synthase (eNOS) and vasodilator-stimulated phosphoprotein (VASP) and decreases apoptosis-related factors Bax, active caspase-9, and active caspase-3 following experimental SAH. Altogether, our results suggest JNJ16259685 improves long-term functional impairment through neurovascular protection.
中文翻译:

负性变构调节剂mGluR1改善了实验性蛛网膜下腔出血后的长期神经功能缺陷。
动脉瘤性蛛网膜下腔出血(SAH)引起永久性神经系统后遗症,但其潜在机制有待进一步阐明。在这里,我们显示负变构调节剂JNJ16259685对代谢型谷氨酸受体1(mGluR1)的抑制作用改善了SAH血管内穿孔模型的长期神经行为结果。JNJ16259685通过抑制大鼠SAH模型中的脑血流量(CBF)减少,脑血管收缩和微血栓形成,改善了脑血管功能障碍。此外,JNJ16259685通过减轻神经元死亡和变性来减少SAH诱导的长期神经元损伤。在机械上,JNJ16259685维持内皮一氧化氮合酶(eNOS)和血管扩张剂刺激的磷蛋白(VASP)的磷酸化,并降低凋亡相关因子Bax,实验性SAH后可激活caspase-9和caspase-3。总之,我们的结果表明JNJ16259685通过神经血管保护改善了长期功能损伤。
更新日期:2020-09-16
中文翻译:

负性变构调节剂mGluR1改善了实验性蛛网膜下腔出血后的长期神经功能缺陷。
动脉瘤性蛛网膜下腔出血(SAH)引起永久性神经系统后遗症,但其潜在机制有待进一步阐明。在这里,我们显示负变构调节剂JNJ16259685对代谢型谷氨酸受体1(mGluR1)的抑制作用改善了SAH血管内穿孔模型的长期神经行为结果。JNJ16259685通过抑制大鼠SAH模型中的脑血流量(CBF)减少,脑血管收缩和微血栓形成,改善了脑血管功能障碍。此外,JNJ16259685通过减轻神经元死亡和变性来减少SAH诱导的长期神经元损伤。在机械上,JNJ16259685维持内皮一氧化氮合酶(eNOS)和血管扩张剂刺激的磷蛋白(VASP)的磷酸化,并降低凋亡相关因子Bax,实验性SAH后可激活caspase-9和caspase-3。总之,我们的结果表明JNJ16259685通过神经血管保护改善了长期功能损伤。


























 京公网安备 11010802027423号
京公网安备 11010802027423号