Journal of Catalysis ( IF 7.3 ) Pub Date : 2020-08-12 , DOI: 10.1016/j.jcat.2020.08.001 Chunzheng Wu , Xinxin Xing , Guang Yang , Tian Tong , Zhiming M. Wang , Jiming Bao
|
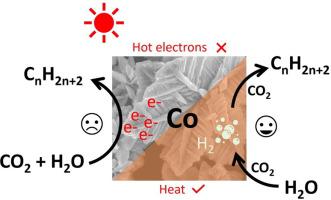
The production of long-chain hydrocarbons from CO2 and water using solar light is a big challenge for the catalysis society. Cobalt nanostructures have been reported to catalyze the production of C3-C15 alkanes in a cobalt-water-CO2 three-phase system under white light, yet the proposed photocatalytic mechanism still lacks evidence. In this work, cobalt micro- and nanostructures with different sizes and morphologies are used as model catalysts to reproduce this reaction. Despite different activity, selectivity and stability, the catalysts are found to follow the same reaction mechanism: a CO2 assisted galvanic replacement reaction to produce H2 followed by a thermal catalytic CO2 hydrogenation to produce alkanes; meanwhile, the Co catalysts are gradually but inevitably converted to CoCO3, leading to a slow deactivation. The low concentration of the in-situ formed H2 favors the growth of C2+ alkanes. The incident light drives the reaction by heating the catalyst, rather than providing plasmonic hot electrons for CO2 reduction.
中文翻译:

了解使用钴纳米结构和光从CO 2和水中生成长链碳氢化合物
利用太阳光从CO 2和水中生产长链碳氢化合物对催化社会是一个巨大的挑战。据报道,钴纳米结构可在白光下在钴-水-CO 2三相系统中催化C3-C15烷烃的产生,但所提出的光催化机理仍缺乏证据。在这项工作中,具有不同尺寸和形态的钴微结构和纳米结构被用作模型催化剂以重现该反应。尽管具有不同的活性,选择性和稳定性,但发现催化剂遵循相同的反应机理:CO 2辅助电置换反应生成H 2,然后进行热催化CO 2氢化产生烷烃;同时,Co催化剂逐渐但不可避免地转化为CoCO 3,导致失活缓慢。低浓度的原位形成的H 2有利于C2 +烷烃的生长。入射光通过加热催化剂而不是提供用于CO 2还原的等离激元热电子来驱动反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号