当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Genetic Code Expansion Enables Site-Specific PEGylation of a Human Growth Hormone Receptor Antagonist through Click Chemistry.
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-08-10 , DOI: 10.1021/acs.bioconjchem.0c00365 Kyle Tamshen 1 , Yue Wang 2 , Stephen M F Jamieson 3, 4 , Jo K Perry 2, 3 , Heather D Maynard 1, 5, 6
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-08-10 , DOI: 10.1021/acs.bioconjchem.0c00365 Kyle Tamshen 1 , Yue Wang 2 , Stephen M F Jamieson 3, 4 , Jo K Perry 2, 3 , Heather D Maynard 1, 5, 6
Affiliation
|
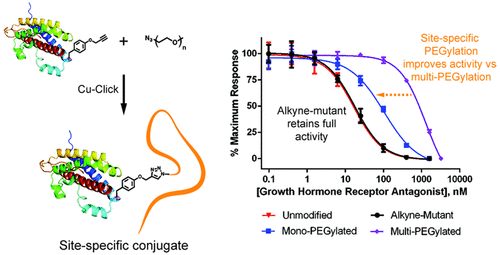
Regulation of human growth hormone (GH) signaling has important applications in the remediation of several diseases including acromegaly and cancer. Growth hormone receptor (GHR) antagonists currently provide the most effective means for suppression of GH signaling. However, these small 22 kDa recombinantly engineered GH analogues exhibit short plasma circulation times. To improve clinical viability, between four and six molecules of 5 kDa poly(ethylene glycol) (PEG) are nonspecifically conjugated to the nine amines of the GHR antagonist designated as B2036 in the FDA-approved therapeutic pegvisomant. PEGylation increases the molecular weight of B2036 and considerably extends its circulation time, but also dramatically reduces its bioactivity, contributing to high dosing requirements and increased cost. As an alternative to nonspecific PEGylation, we report the use of genetic code expansion technology to site-specifically incorporate the unnatural amino acid propargyl tyrosine (pglY) into B2036 with the goal of producing site-specific protein–polymer conjugates. Substitution of tyrosine 35 with pglY yielded a B2036 variant containing an alkyne functional group without compromising bioactivity, as verified by a cellular assay. Subsequent conjugation of 5, 10, and 20 kDa azide-containing PEGs via the copper-catalyzed click reaction yielded high purity, site-specific conjugates with >89% conjugation efficiencies. Site-specific attachment of PEG to B2036 is associated with substantially improved in vitro bioactivity values compared to pegvisomant, with an inverse relationship between polymer size and activity observed. Notably, the B2036-20 kDa PEG conjugate has a molecular weight comparable to pegvisomant, while exhibiting a 12.5 fold improvement in half-maximal inhibitory concentration in GHR-expressing Ba/F3 cells (103.3 nM vs 1289 nM). We expect that this straightforward route to achieve site-specific GHR antagonists will be useful for GH signal regulation.
中文翻译:

遗传密码扩展通过点击化学实现了人类生长激素受体拮抗剂的位点特异性聚乙二醇化。
人类生长激素 (GH) 信号的调节在包括肢端肥大症和癌症在内的多种疾病的治疗中具有重要应用。生长激素受体 (GHR) 拮抗剂目前是抑制 GH 信号传导的最有效手段。然而,这些小的 22 kDa 重组工程 GH 类似物表现出较短的血浆循环时间。为了提高临床可行性,将 4 到 6 个 5 kDa 聚乙二醇 (PEG) 分子非特异性偶联到 GHR 拮抗剂的 9 种胺上,该拮抗剂在 FDA 批准的治疗性培维索孟中被指定为 B2036。聚乙二醇化增加了 B2036 的分子量并显着延长了其循环时间,但也显着降低了其生物活性,导致高剂量要求和成本增加。作为非特异性聚乙二醇化的替代方法,我们报告了使用遗传密码扩展技术将非天然氨基酸炔丙基酪氨酸 (pglY) 位点特异性地整合到 B2036 中,目的是生产位点特异性蛋白质-聚合物偶联物。用 pglY 取代酪氨酸 35 产生了 B2036 变体,该变体含有炔官能团而不影响生物活性,如细胞测定所证实的那样。随后通过铜催化的点击反应结合含有 5、10 和 20 kDa 叠氮化物的 PEG,产生高纯度、位点特异性结合物,结合效率 >89%。与培维索孟相比,PEG 与 B2036 的位点特异性连接与显着改善的体外生物活性值相关,观察到聚合物大小和活性之间呈反比关系。尤其,B2036-20 kDa PEG 偶联物的分子量与培维索孟相当,同时在表达 GHR 的 Ba/F3 细胞中的半数最大抑制浓度提高了 12.5 倍(103.3 nM 对 1289 nM)。我们预计这种实现位点特异性 GHR 拮抗剂的直接途径将对 GH 信号调节有用。
更新日期:2020-09-16
中文翻译:

遗传密码扩展通过点击化学实现了人类生长激素受体拮抗剂的位点特异性聚乙二醇化。
人类生长激素 (GH) 信号的调节在包括肢端肥大症和癌症在内的多种疾病的治疗中具有重要应用。生长激素受体 (GHR) 拮抗剂目前是抑制 GH 信号传导的最有效手段。然而,这些小的 22 kDa 重组工程 GH 类似物表现出较短的血浆循环时间。为了提高临床可行性,将 4 到 6 个 5 kDa 聚乙二醇 (PEG) 分子非特异性偶联到 GHR 拮抗剂的 9 种胺上,该拮抗剂在 FDA 批准的治疗性培维索孟中被指定为 B2036。聚乙二醇化增加了 B2036 的分子量并显着延长了其循环时间,但也显着降低了其生物活性,导致高剂量要求和成本增加。作为非特异性聚乙二醇化的替代方法,我们报告了使用遗传密码扩展技术将非天然氨基酸炔丙基酪氨酸 (pglY) 位点特异性地整合到 B2036 中,目的是生产位点特异性蛋白质-聚合物偶联物。用 pglY 取代酪氨酸 35 产生了 B2036 变体,该变体含有炔官能团而不影响生物活性,如细胞测定所证实的那样。随后通过铜催化的点击反应结合含有 5、10 和 20 kDa 叠氮化物的 PEG,产生高纯度、位点特异性结合物,结合效率 >89%。与培维索孟相比,PEG 与 B2036 的位点特异性连接与显着改善的体外生物活性值相关,观察到聚合物大小和活性之间呈反比关系。尤其,B2036-20 kDa PEG 偶联物的分子量与培维索孟相当,同时在表达 GHR 的 Ba/F3 细胞中的半数最大抑制浓度提高了 12.5 倍(103.3 nM 对 1289 nM)。我们预计这种实现位点特异性 GHR 拮抗剂的直接途径将对 GH 信号调节有用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号