当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism Underlying Anti-Markovnikov Addition in the Reaction of Pentalenene Synthase.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-10 , DOI: 10.1021/acs.biochem.0c00518 Jason O Matos 1 , Ramasamy P Kumar 1 , Alison C Ma 1 , MacKenzie Patterson 1 , Isaac J Krauss 2 , Daniel D Oprian 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-10 , DOI: 10.1021/acs.biochem.0c00518 Jason O Matos 1 , Ramasamy P Kumar 1 , Alison C Ma 1 , MacKenzie Patterson 1 , Isaac J Krauss 2 , Daniel D Oprian 1
Affiliation
|
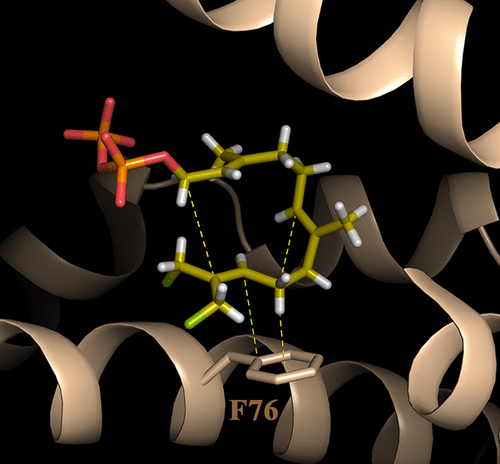
Most terpene synthase reactions follow Markovnikov rules for formation of high-energy carbenium ion intermediates. However, there are notable exceptions. For example, pentalenene synthase (PS) undergoes an initial anti-Markovnikov cyclization reaction followed by a 1,2-hydride shift to form an intermediate humulyl cation with positive charge on the secondary carbon C9 atom of the farnesyl diphosphate substrate. The mechanism by which these enzymes stabilize and guide the regioselectivity of secondary carbocations has not heretofore been elucidated. In an effort to better understand these reactions, we grew crystals of apo-PS, soaked them with the nonreactive substrate analogue 12,13-difluorofarnesyl diphosphate, and determined the X-ray structure of the resulting complex at 2.2 Å resolution. The most striking feature of the active site structure is that C9 is perfectly positioned to make a C–H···π interaction with the side chain benzene ring of residue F76; this would enhance hyperconjugation to stabilize a developing cation at C10 and thus support the anti-Markovnikov regioselectivity of the cyclization. The benzene ring is also positioned to catalyze the migration of H to C10 and stabilize a C9 carbocation. On the opposite face of C9, further cation stabilization is possible via interactions with the main chain carbonyl of I177 and the neighboring intramolecular C6═C7 bond. Mutagenesis experiments also support a role for residue 76 in these interactions, but most interesting is the F76W mutant, whose crystal structure clearly shows C9 and C10 centered above the fused benzene and pyrrole rings of the indole side chain, respectively, such that a carbocation at either position could be stabilized in this complex, and two anti-Markovnikov products, pentalenene and humulene, are formed. Finally, we show that there is a rough correlation (although not absolute) of an aromatic side chain (F or Y) at position 76 in related terpene synthases from Streptomyces that catalyze similar anti-Markovnikov addition reactions.
中文翻译:

戊烯合酶反应中反马尔科夫尼科夫加成的潜在机制。
大多数萜烯合酶反应遵循马尔科夫尼科夫规则,以形成高能碳正离子中间体。但是,也有明显的例外。例如,戊烯合酶 (PS) 经历初始反马尔科夫尼科夫环化反应,然后发生 1,2-氢化物转移,在法呢基二磷酸底物的仲碳 C9 原子上形成带正电荷的中间腐殖酸阳离子。这些酶稳定和引导二级碳正离子的区域选择性的机制迄今尚未阐明。为了更好地理解这些反应,我们生长了 apo-PS 晶体,用非反应性底物类似物 12,13-二氟法呢基二磷酸浸泡它们,并以 2.2 Å 分辨率确定所得复合物的 X 射线结构。活性位点结构最显着的特点是C9完美定位,与残基F76的侧链苯环发生C-H···π相互作用;这将增强超共轭以稳定 C10 处的发展中的阳离子,从而支持环化的反马尔科夫尼科夫区域选择性。苯环也定位为催化 H 迁移到 C10 并稳定 C9 碳正离子。在 C9 的相反面上,通过与 I177 的主链羰基和相邻的分子内 C6=C7 键的相互作用,可以进一步稳定阳离子。诱变实验也支持残基 76 在这些相互作用中的作用,但最有趣的是 F76W 突变体,其晶体结构清楚地显示 C9 和 C10 分别位于吲哚侧链的稠合苯和吡咯环上方,使得该复合物中任一位置的碳正离子都可以稳定,并形成两种反马尔科夫尼科夫产物,戊烯和葎烯。最后,我们表明在相关萜烯合酶的第 76 位存在一个粗略的相关性(尽管不是绝对的)芳香侧链(F 或 Y)催化类似抗马尔科夫尼科夫加成反应的链霉菌。
更新日期:2020-09-08
中文翻译:

戊烯合酶反应中反马尔科夫尼科夫加成的潜在机制。
大多数萜烯合酶反应遵循马尔科夫尼科夫规则,以形成高能碳正离子中间体。但是,也有明显的例外。例如,戊烯合酶 (PS) 经历初始反马尔科夫尼科夫环化反应,然后发生 1,2-氢化物转移,在法呢基二磷酸底物的仲碳 C9 原子上形成带正电荷的中间腐殖酸阳离子。这些酶稳定和引导二级碳正离子的区域选择性的机制迄今尚未阐明。为了更好地理解这些反应,我们生长了 apo-PS 晶体,用非反应性底物类似物 12,13-二氟法呢基二磷酸浸泡它们,并以 2.2 Å 分辨率确定所得复合物的 X 射线结构。活性位点结构最显着的特点是C9完美定位,与残基F76的侧链苯环发生C-H···π相互作用;这将增强超共轭以稳定 C10 处的发展中的阳离子,从而支持环化的反马尔科夫尼科夫区域选择性。苯环也定位为催化 H 迁移到 C10 并稳定 C9 碳正离子。在 C9 的相反面上,通过与 I177 的主链羰基和相邻的分子内 C6=C7 键的相互作用,可以进一步稳定阳离子。诱变实验也支持残基 76 在这些相互作用中的作用,但最有趣的是 F76W 突变体,其晶体结构清楚地显示 C9 和 C10 分别位于吲哚侧链的稠合苯和吡咯环上方,使得该复合物中任一位置的碳正离子都可以稳定,并形成两种反马尔科夫尼科夫产物,戊烯和葎烯。最后,我们表明在相关萜烯合酶的第 76 位存在一个粗略的相关性(尽管不是绝对的)芳香侧链(F 或 Y)催化类似抗马尔科夫尼科夫加成反应的链霉菌。


























 京公网安备 11010802027423号
京公网安备 11010802027423号