当前位置:
X-MOL 学术
›
Vib. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural and spectroscopic investigation of the chalcones (E)-1-(4-aminophenyl)-3-(4’-ethoxyphenyl)-prop-2-en-1-one and (E)-1-(aminophenyl)-3-(4’-methoxyphenyl)-prop-2-en-1-one
Vibrational Spectroscopy ( IF 2.5 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.vibspec.2020.103118 Igor Kleber Campos Lima , Filipe Dantas de Sousa , Ana Joyce de Morais Bento , Beatriz Gonçalves Cruz , Priscila Teixeira da Silva , Paulo Nogueira Bandeira , Hélcio Silva dos Santos , Gilberto Dantas Saraiva , Antônio César Honorato Barreto , Paulo de Tarso Cavalcante Freire , Alexandre Magno Rodrigues Teixeira
Vibrational Spectroscopy ( IF 2.5 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.vibspec.2020.103118 Igor Kleber Campos Lima , Filipe Dantas de Sousa , Ana Joyce de Morais Bento , Beatriz Gonçalves Cruz , Priscila Teixeira da Silva , Paulo Nogueira Bandeira , Hélcio Silva dos Santos , Gilberto Dantas Saraiva , Antônio César Honorato Barreto , Paulo de Tarso Cavalcante Freire , Alexandre Magno Rodrigues Teixeira
|
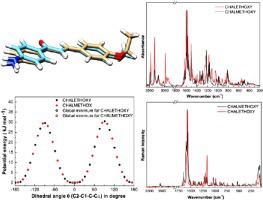
ABSTRACT In this study, two 4′-aminochalcones with structural similarity were structurally characterized using the NMR technique of 1H and 13C. Polycrystalline samples of these chalcones were analyzed by FT-Raman and ATR-FTIR spectroscopy at room temperature in the regions 40-4000 cm-1 and 400-4000 cm-1, respectively. The DFT calculations using the B3LYP functional with 6-311 G(d,p) basis set were performed, revealing the optimized molecular geometry and the vibrational wavenumbers for these chalcones. Conformational analysis was done from the optimized structures to obtain different conformers. The calculated harmonic vibrational wavenumbers were scaled to improve the agreement between theoretical and experimental wavenumbers. A complete vibrational assignment using Potential Energy Distributions (PED) was provided for the observed Raman and infrared spectra. The scaled wavenumbers were found to be in good agreement with experimental wavenumbers. Furthermore, a comparative structural and vibrational analysis was performed. The structural analysis allowed us concluding that in addition to differences in the position 4′ – where one chalcone has a methoxy group and the other, an ethoxy group – the molecular conformation of them is significantly different, especially regarding the torsion angles linking the enone chains with the rings of these chalcones. The vibrational analysis allowed us to identify all Raman and infrared bands of the two chalcones, highlighting the main differences and correspondences between them.
中文翻译:

查耳酮 (E)-1-(4-氨基苯基)-3-(4'-乙氧基苯基)-prop-2-en-1-one 和 (E)-1-(氨基苯基)-3- 的结构和光谱研究(4'-甲氧基苯基)-prop-2-en-1-one
摘要 在本研究中,使用 1H 和 13C 的 NMR 技术对结构相似的两个 4'-氨基查耳酮进行了结构表征。这些查耳酮的多晶样品分别在室温下在 40-4000 cm-1 和 400-4000 cm-1 区域通过 FT-拉曼和 ATR-FTIR 光谱分析。使用具有 6-311 G(d,p) 基组的 B3LYP 函数进行 DFT 计算,揭示了这些查耳酮的优化分子几何结构和振动波数。从优化的结构进行构象分析以获得不同的构象异构体。计算的谐波振动波数被缩放以提高理论和实验波数之间的一致性。为观察到的拉曼光谱和红外光谱提供了使用势能分布 (PED) 的完整振动分配。发现缩放的波数与实验波数非常一致。此外,还进行了比较结构和振动分析。结构分析使我们得出结论,除了 4' 位的差异(其中一个查耳酮具有甲氧基而另一个具有乙氧基)之外,它们的分子构象也存在显着差异,尤其是在连接烯酮链的扭转角方面与这些查耳酮的戒指。振动分析使我们能够识别两个查耳酮的所有拉曼和红外波段,突出了它们之间的主要差异和对应关系。发现缩放的波数与实验波数非常一致。此外,还进行了比较结构和振动分析。结构分析使我们得出结论,除了 4' 位的差异(其中一个查耳酮具有甲氧基而另一个具有乙氧基)之外,它们的分子构象也存在显着差异,尤其是在连接烯酮链的扭转角方面与这些查耳酮的戒指。振动分析使我们能够识别两个查耳酮的所有拉曼和红外波段,突出了它们之间的主要差异和对应关系。发现缩放的波数与实验波数非常一致。此外,还进行了比较结构和振动分析。结构分析使我们得出结论,除了 4' 位的差异(其中一个查耳酮具有甲氧基而另一个具有乙氧基)之外,它们的分子构象也存在显着差异,尤其是在连接烯酮链的扭转角方面与这些查耳酮的戒指。振动分析使我们能够识别两个查耳酮的所有拉曼和红外波段,突出了它们之间的主要差异和对应关系。结构分析使我们得出结论,除了 4' 位的差异(其中一个查耳酮具有甲氧基而另一个具有乙氧基)之外,它们的分子构象也存在显着差异,尤其是在连接烯酮链的扭转角方面与这些查耳酮的戒指。振动分析使我们能够识别两个查耳酮的所有拉曼和红外波段,突出了它们之间的主要差异和对应关系。结构分析使我们得出结论,除了 4' 位的差异(其中一个查耳酮具有甲氧基而另一个具有乙氧基)之外,它们的分子构象也存在显着差异,尤其是在连接烯酮链的扭转角方面与这些查耳酮的戒指。振动分析使我们能够识别两个查耳酮的所有拉曼和红外波段,突出了它们之间的主要差异和对应关系。
更新日期:2020-09-01
中文翻译:

查耳酮 (E)-1-(4-氨基苯基)-3-(4'-乙氧基苯基)-prop-2-en-1-one 和 (E)-1-(氨基苯基)-3- 的结构和光谱研究(4'-甲氧基苯基)-prop-2-en-1-one
摘要 在本研究中,使用 1H 和 13C 的 NMR 技术对结构相似的两个 4'-氨基查耳酮进行了结构表征。这些查耳酮的多晶样品分别在室温下在 40-4000 cm-1 和 400-4000 cm-1 区域通过 FT-拉曼和 ATR-FTIR 光谱分析。使用具有 6-311 G(d,p) 基组的 B3LYP 函数进行 DFT 计算,揭示了这些查耳酮的优化分子几何结构和振动波数。从优化的结构进行构象分析以获得不同的构象异构体。计算的谐波振动波数被缩放以提高理论和实验波数之间的一致性。为观察到的拉曼光谱和红外光谱提供了使用势能分布 (PED) 的完整振动分配。发现缩放的波数与实验波数非常一致。此外,还进行了比较结构和振动分析。结构分析使我们得出结论,除了 4' 位的差异(其中一个查耳酮具有甲氧基而另一个具有乙氧基)之外,它们的分子构象也存在显着差异,尤其是在连接烯酮链的扭转角方面与这些查耳酮的戒指。振动分析使我们能够识别两个查耳酮的所有拉曼和红外波段,突出了它们之间的主要差异和对应关系。发现缩放的波数与实验波数非常一致。此外,还进行了比较结构和振动分析。结构分析使我们得出结论,除了 4' 位的差异(其中一个查耳酮具有甲氧基而另一个具有乙氧基)之外,它们的分子构象也存在显着差异,尤其是在连接烯酮链的扭转角方面与这些查耳酮的戒指。振动分析使我们能够识别两个查耳酮的所有拉曼和红外波段,突出了它们之间的主要差异和对应关系。发现缩放的波数与实验波数非常一致。此外,还进行了比较结构和振动分析。结构分析使我们得出结论,除了 4' 位的差异(其中一个查耳酮具有甲氧基而另一个具有乙氧基)之外,它们的分子构象也存在显着差异,尤其是在连接烯酮链的扭转角方面与这些查耳酮的戒指。振动分析使我们能够识别两个查耳酮的所有拉曼和红外波段,突出了它们之间的主要差异和对应关系。结构分析使我们得出结论,除了 4' 位的差异(其中一个查耳酮具有甲氧基而另一个具有乙氧基)之外,它们的分子构象也存在显着差异,尤其是在连接烯酮链的扭转角方面与这些查耳酮的戒指。振动分析使我们能够识别两个查耳酮的所有拉曼和红外波段,突出了它们之间的主要差异和对应关系。结构分析使我们得出结论,除了 4' 位的差异(其中一个查耳酮具有甲氧基而另一个具有乙氧基)之外,它们的分子构象也存在显着差异,尤其是在连接烯酮链的扭转角方面与这些查耳酮的戒指。振动分析使我们能够识别两个查耳酮的所有拉曼和红外波段,突出了它们之间的主要差异和对应关系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号