Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-08-11 , DOI: 10.1016/j.jmb.2020.08.005 Yifan Zhang 1 , Katherine A Edmonds 2 , Daniel J Raines 3 , Brennan A Murphy 2 , Hongwei Wu 2 , Chuchu Guo 4 , Elizabeth M Nolan 4 , Michael S VanNieuwenhze 2 , Anne-K Duhme-Klair 3 , David P Giedroc 1
|
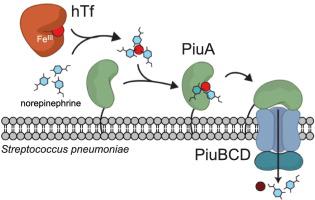
Streptococcus pneumoniae (Spn) is an important Gram-positive human pathogen that causes millions of infections worldwide with an increasing occurrence of antibiotic resistance. Fe acquisition is a crucial virulence determinant in Spn; further, Spn relies on exogenous FeIII-siderophore scavenging to meet nutritional Fe needs. Recent studies suggest that the human catecholamine stress hormone, norepinephrine (NE), facilitates Fe acquisition in Spn under conditions of transferrin-mediated Fe starvation. Here we show that the solute binding lipoprotein PiuA from the piu Fe acquisition ABC transporter PiuBCDA, previously described as an Fe-hemin binding protein, binds tetradentate catechol FeIII complexes, including NE and the hydrolysis products of enterobactin. Two protein-derived ligands (H238, Y300) create a coordinately saturated FeIII complex, which parallel recent studies in the Gram-negative intestinal pathogen Campylobacter jejuni. Our in vitro studies using NMR spectroscopy and 54Fe LC-ICP-MS confirm the FeIII can move from transferrin to apo-PiuA in an NE-dependent manner. Structural analysis of PiuA FeIII-bis-catechol and GaIII-bis-catechol and GaIII-(NE)2 complexes by NMR spectroscopy reveals only localized structural perturbations in PiuA upon ligand binding, largely consistent with recent descriptions of other solute binding proteins of type II ABC transporters. We speculate that tetradentate FeIII complexes formed by mono- and bis-catechol species are important Fe sources in Gram-positive human pathogens, since PiuA functions in the same way as SstD from Staphylococcus aureus.
中文翻译:

肺炎球菌铁摄取蛋白 A (PiuA) 特别识别四齿 FeIIIbis 和单儿茶酚复合物。
肺炎链球菌 (Spn)是一种重要的革兰氏阳性人类病原体,在全球范围内引起数百万感染,抗生素耐药性的发生率不断增加。Fe 的获取是Spn 中一个关键的毒力决定因素;此外,Spn依靠外源性 Fe III -铁载体清除来满足营养性 Fe 需求。最近的研究表明,人儿茶酚胺应激激素去甲肾上腺素 (NE) 在转铁蛋白介导的 Fe 饥饿条件下促进Spn 中的Fe 获取。在这里,我们展示了来自piu Fe 采集 ABC 转运蛋白 PiuBCDA 的溶质结合脂蛋白 PiuA ,以前描述为 Fe-hemin 结合蛋白,结合四齿儿茶酚 FeIII复合物,包括 NE 和肠杆菌素的水解产物。两种蛋白质衍生的配体(H238、Y300)产生了一个协调饱和的 Fe III复合物,这与最近在革兰氏阴性肠道病原体空肠弯曲杆菌中的研究相似。我们使用 NMR 光谱和54 Fe LC-ICP-MS进行的体外研究证实,Fe III可以以依赖于 NE 的方式从转铁蛋白移动到 apo-PiuA。PiuA Fe III - bis -catechol 和 Ga III - bis -catechol 和 Ga III -(NE) 2 的结构分析通过 NMR 光谱分析的复合物仅在配体结合时显示 PiuA 中的局部结构扰动,这与最近对 II 型 ABC 转运蛋白的其他溶质结合蛋白的描述基本一致。我们推测由单和双儿茶酚物种形成的四齿 Fe III复合物是革兰氏阳性人类病原体中重要的 Fe 来源,因为 PiuA 的功能与来自金黄色葡萄球菌的SstD 相同。


























 京公网安备 11010802027423号
京公网安备 11010802027423号