当前位置:
X-MOL 学术
›
Med. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Propargylated monocarbonyl curcumin analogues: synthesis, bioevaluation and molecular docking study
Medicinal Chemistry Research ( IF 2.6 ) Pub Date : 2020-08-10 , DOI: 10.1007/s00044-020-02611-7 Amol A. Nagargoje , Satish V. Akolkar , Dnyaneshwar D. Subhedar , Mubarak H. Shaikh , Jaiprakash N. Sangshetti , Vijay M. Khedkar , Bapurao B. Shingate
Medicinal Chemistry Research ( IF 2.6 ) Pub Date : 2020-08-10 , DOI: 10.1007/s00044-020-02611-7 Amol A. Nagargoje , Satish V. Akolkar , Dnyaneshwar D. Subhedar , Mubarak H. Shaikh , Jaiprakash N. Sangshetti , Vijay M. Khedkar , Bapurao B. Shingate
In the current experimental study, we have synthesised new monocarbonyl curcumin analogues bearing propargyl ether moiety in their structure and evaluated for in vitro antifungal and radical scavenging activity. The antifungal activity was carried out against five human pathogenic fungal strains such as Candida albicans, Fusarium oxysporum, Aspergillus flavus, Aspergillus niger and Cryptococcus neoformans. Most of the curcumin analogues displayed excellent to moderate fungicidal activity when compared with standard drug Miconazole. Also, synthesised analogues exhibited potential radical scavenging activity as compared with standard antioxidant Butylated hydroxyl toluene (BHT). Based on biological data, structure-activity relationship (SAR) were also discussed. Furthermore, in silico computational study was carried out to know binding interactions of synthesised analogues in the active sites of enzyme sterol 14α-demethylase (CYP51).
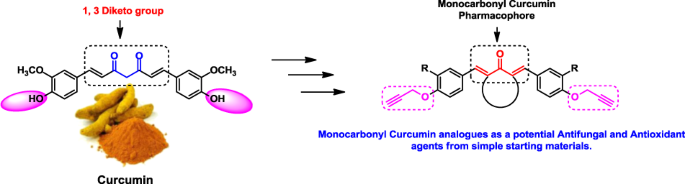
中文翻译:

炔丙基单羰基姜黄素类似物:合成,生物评价和分子对接研究
在当前的实验研究中,我们合成了在结构中带有炔丙基醚部分的新单羰基姜黄素类似物,并评估了其体外抗真菌和自由基清除活性。对五种白色念珠菌,尖孢镰刀菌,黄曲霉,黑曲霉和新隐球菌等五种人类致病真菌菌株进行了抗真菌活性。。与标准药物咪康唑相比,大多数姜黄素类似物均表现出优异至中等的杀菌活性。此外,与标准抗氧化剂丁基化羟基甲苯(BHT)相比,合成的类似物还具有潜在的自由基清除活性。基于生物学数据,还讨论了结构-活性关系(SAR)。此外,进行了计算机计算研究以了解合成的类似物在固醇14α-脱甲基酶(CYP51)活性位点的结合相互作用。
更新日期:2020-08-10

中文翻译:

炔丙基单羰基姜黄素类似物:合成,生物评价和分子对接研究
在当前的实验研究中,我们合成了在结构中带有炔丙基醚部分的新单羰基姜黄素类似物,并评估了其体外抗真菌和自由基清除活性。对五种白色念珠菌,尖孢镰刀菌,黄曲霉,黑曲霉和新隐球菌等五种人类致病真菌菌株进行了抗真菌活性。。与标准药物咪康唑相比,大多数姜黄素类似物均表现出优异至中等的杀菌活性。此外,与标准抗氧化剂丁基化羟基甲苯(BHT)相比,合成的类似物还具有潜在的自由基清除活性。基于生物学数据,还讨论了结构-活性关系(SAR)。此外,进行了计算机计算研究以了解合成的类似物在固醇14α-脱甲基酶(CYP51)活性位点的结合相互作用。




























 京公网安备 11010802027423号
京公网安备 11010802027423号