当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NIS-promoted intermolecular bis-sulfenylation of allenamides via a two-step radical process: synthesis of 1,3-dithioethers
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-08-10 , DOI: 10.1039/d0qo00690d Xiao Yuan 1, 2, 3, 4, 5 , Xiaoju Tan 1, 2, 3, 4, 5 , Na Ding 1, 2, 3, 4, 5 , Yongchun Liu 1, 2, 3, 4, 5 , Xiaoxiao Li 1, 2, 3, 4, 5 , Zhigang Zhao 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-08-10 , DOI: 10.1039/d0qo00690d Xiao Yuan 1, 2, 3, 4, 5 , Xiaoju Tan 1, 2, 3, 4, 5 , Na Ding 1, 2, 3, 4, 5 , Yongchun Liu 1, 2, 3, 4, 5 , Xiaoxiao Li 1, 2, 3, 4, 5 , Zhigang Zhao 1, 2, 3, 4, 5
Affiliation
|
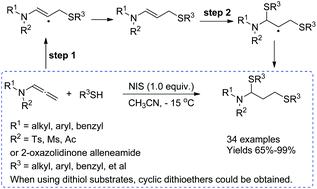
An efficient NIS-promoted two-step radical addition of thiols to allenamides was developed to create a workable route to 1,3-dithioethers. The reaction exhibited good functional group tolerance and high efficiency, affording the products in good to excellent yields. Moreover, cyclic dithioethers could be obtained by using 1,2-dithiols as substrates. Mechanistic investigations including calculation study indicated that a two-step radical process is likely involved in this transformation.
中文翻译:

NIS通过两步自由基过程促进烯丙酰胺的分子间双亚磺基化:合成1,3-二硫醚
已开发出一种有效的NIS促进的将硫醇两步自由基加成到烯丙基酰胺上的方法,以创建通往1,3-二硫醚的可行途径。该反应显示出良好的官能团耐受性和高效率,从而提供了良好至优异产率的产物。此外,通过使用1,2-二硫醇作为底物可以获得环状二硫醚。包括计算研究在内的机械研究表明,这一转变可能涉及两步根本过程。
更新日期:2020-09-16
中文翻译:

NIS通过两步自由基过程促进烯丙酰胺的分子间双亚磺基化:合成1,3-二硫醚
已开发出一种有效的NIS促进的将硫醇两步自由基加成到烯丙基酰胺上的方法,以创建通往1,3-二硫醚的可行途径。该反应显示出良好的官能团耐受性和高效率,从而提供了良好至优异产率的产物。此外,通过使用1,2-二硫醇作为底物可以获得环状二硫醚。包括计算研究在内的机械研究表明,这一转变可能涉及两步根本过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号