当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Immunogenicity of Antigen Adjuvanted with AS04 and Its Deposition in the Upper Respiratory Tract after Intranasal Administration.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-08-10 , DOI: 10.1021/acs.molpharmaceut.0c00372 Haiyue Xu 1 , Riyad F Alzhrani 1 , Zachary N Warnken 1 , Sachin G Thakkar 1 , Mingtao Zeng 2 , Hugh D C Smyth 1 , Robert O Williams 1 , Zhengrong Cui 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-08-10 , DOI: 10.1021/acs.molpharmaceut.0c00372 Haiyue Xu 1 , Riyad F Alzhrani 1 , Zachary N Warnken 1 , Sachin G Thakkar 1 , Mingtao Zeng 2 , Hugh D C Smyth 1 , Robert O Williams 1 , Zhengrong Cui 1
Affiliation
|
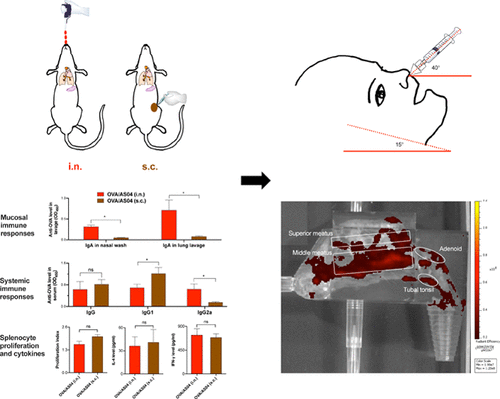
Adjuvant system 04 (AS04) is in injectable human vaccines. AS04 contains two known adjuvants, 3-O-desacyl-4′-monophosphoryl lipid A (MPL) and insoluble aluminum salts. Data from previous studies showed that both MPL and insoluble aluminum salts have nasal mucosal vaccine adjuvant activity. The present study was designed to test the feasibility of using AS04 as an adjuvant to help nasally administered antigens to induce specific mucosal and systemic immunity as well as to evaluate the deposition of antigens in the upper respiratory tract when adjuvanted with AS04. Alhydrogel, an aluminum (oxy)hydroxide suspension, was mixed with MPL to form AS04, which was then mixed with ovalbumin (OVA) or 3× M2e-HA2, a synthetic influenza virus hemagglutinin fusion protein, as an antigen to prepare OVA/AS04 and 3× M2e-HA2/AS04 vaccines, respectively. In mice, AS04 enabled antigens, when given intranasally, to induce specific IgA response in nasal and lung mucosal secretions as well as specific IgG response in the serum samples of the immunized mice, whereas subcutaneous injection of the same vaccine induced specific antibody responses only in the serum samples but not in the mucosal secretions. Splenocytes isolated from mice intranasally immunized with the OVA/AS04 also proliferated and released cytokines (i.e., IL-4 and IFN-γ) after in vitro stimulation with the antigen. In the immunogenicity test, intranasal OVA/AS04 was not more effective than intranasal OVA/MPL at the dosing regimens tested. However, when compared to OVA/MPL, OVA/AS04 showed a different atomized droplet size distribution and more importantly a more favorable OVA deposition profile when atomized into a nasal cast that was 3-D printed based on the computer tomography scan of the nose of a child. It is concluded that AS04 has mucosal adjuvant activity when given intranasally. In addition, there is a reason to be optimistic about using AS04 as an adjuvant to target an antigen of interest to the right region of the nasal cavity in humans for immune response induction.
中文翻译:

鼻内给药后,AS04佐剂抗原的免疫原性及其在上呼吸道的沉积。
佐剂系统04(AS04)用于可注射的人类疫苗。AS04包含两种已知的佐剂3- O-去酰基-4'-单磷酰基脂质A(MPL)和不溶性铝盐。先前研究的数据表明,MPL和不溶性铝盐均具有鼻粘膜疫苗佐剂活性。本研究旨在测试使用AS04作为佐剂的可行性,以帮助鼻腔给药的抗原诱导特异性粘膜和全身免疫,并评估与AS04佐剂结合时抗原在上呼吸道中的沉积。将Alhydrogel(氢氧化铝)悬浮液与MPL混合以形成AS04,然后将其与卵清蛋白(OVA)或3×M2e-HA2(一种合成的流感病毒血凝素融合蛋白)混合并作为抗原制备OVA / AS04和3x M2e-HA2 / AS04疫苗。在小鼠中,鼻腔给予AS04的抗原,在鼻和肺粘膜分泌物中诱导特异性IgA反应,以及在免疫小鼠血清样品中诱导特异性IgG反应,而皮下注射相同疫苗仅在血清样品中诱导特异性抗体反应,而在粘膜分泌物中则不诱导特异性抗体反应。从经OVA / AS04鼻内免疫的小鼠中分离出的脾细胞也增殖并释放细胞因子(抗原体外刺激后,即IL-4和IFN-γ)。在免疫原性测试中,在测试的给药方案中,鼻内OVA / AS04的效果不及鼻内OVA / MPL。但是,与OVA / MPL相比,OVA / AS04在雾化成基于3D鼻子鼻子的3D打印的鼻腔铸型时,雾化的液滴尺寸分布不同,更重要的是OVA沉积轮廓更佳。一个孩子。结论是鼻内给予AS04具有粘膜佐剂活性。另外,有理由对使用AS04作为佐剂将目标抗原靶向人鼻腔的右侧区域以进行免疫应答诱导感到乐观。
更新日期:2020-09-09
中文翻译:

鼻内给药后,AS04佐剂抗原的免疫原性及其在上呼吸道的沉积。
佐剂系统04(AS04)用于可注射的人类疫苗。AS04包含两种已知的佐剂3- O-去酰基-4'-单磷酰基脂质A(MPL)和不溶性铝盐。先前研究的数据表明,MPL和不溶性铝盐均具有鼻粘膜疫苗佐剂活性。本研究旨在测试使用AS04作为佐剂的可行性,以帮助鼻腔给药的抗原诱导特异性粘膜和全身免疫,并评估与AS04佐剂结合时抗原在上呼吸道中的沉积。将Alhydrogel(氢氧化铝)悬浮液与MPL混合以形成AS04,然后将其与卵清蛋白(OVA)或3×M2e-HA2(一种合成的流感病毒血凝素融合蛋白)混合并作为抗原制备OVA / AS04和3x M2e-HA2 / AS04疫苗。在小鼠中,鼻腔给予AS04的抗原,在鼻和肺粘膜分泌物中诱导特异性IgA反应,以及在免疫小鼠血清样品中诱导特异性IgG反应,而皮下注射相同疫苗仅在血清样品中诱导特异性抗体反应,而在粘膜分泌物中则不诱导特异性抗体反应。从经OVA / AS04鼻内免疫的小鼠中分离出的脾细胞也增殖并释放细胞因子(抗原体外刺激后,即IL-4和IFN-γ)。在免疫原性测试中,在测试的给药方案中,鼻内OVA / AS04的效果不及鼻内OVA / MPL。但是,与OVA / MPL相比,OVA / AS04在雾化成基于3D鼻子鼻子的3D打印的鼻腔铸型时,雾化的液滴尺寸分布不同,更重要的是OVA沉积轮廓更佳。一个孩子。结论是鼻内给予AS04具有粘膜佐剂活性。另外,有理由对使用AS04作为佐剂将目标抗原靶向人鼻腔的右侧区域以进行免疫应答诱导感到乐观。



























 京公网安备 11010802027423号
京公网安备 11010802027423号