当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Determination and Correlation of the Solubility of d(−)-Salicin in Pure and Binary Solvent Systems
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-08-10 , DOI: 10.1021/acs.jced.0c00332 Haishuang Huang 1 , Jingxuan Qiu 1 , Hui He 1 , Dengjing Yi 1, 2 , Mengyao An 2, 3 , Haoyou Liu 1, 2 , Shen Hu 1, 2 , Jiaming Han 1, 2 , Ying Guo 1, 2 , Ning Wei 1 , Peng Wang 1, 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-08-10 , DOI: 10.1021/acs.jced.0c00332 Haishuang Huang 1 , Jingxuan Qiu 1 , Hui He 1 , Dengjing Yi 1, 2 , Mengyao An 2, 3 , Haoyou Liu 1, 2 , Shen Hu 1, 2 , Jiaming Han 1, 2 , Ying Guo 1, 2 , Ning Wei 1 , Peng Wang 1, 2
Affiliation
|
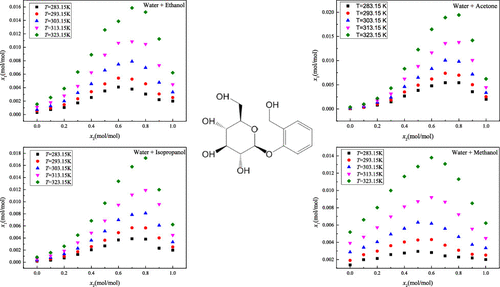
Experimental solubility data of d(−)-salicin in twelve neat solvents (methanol, ethanol, n-propanol, isopropanol, n-butanol, isobutanol, n-pentanol, acetone, acetonitrile, 1,4-dioxance, ethyl acetate, and water) and four binary solvents (water + ethanol, water + acetone, water + isopropanol, and water + methanol) in the temperature range from 283.15 to 323.15 K under atmosphere pressure were determined using the gravimetric method. When the solvent composition was constant, a positive correlation of d(−)-salicin solubility with experimental temperature was found in all investigated pure and mixed solvent systems. At a given temperature, inflection points were found in the solubility curves for binary solvent systems as the mole fraction of water increased, which may be attributed to the cosolvency phenomenon. For the monosolvents, the solubility of d(−)-salicin may be affected by certain factors, such as polarity, steric effects, dipolarity–polarizability, hydrogen bonds, and so on. The KAT-LSER model was used to perform the multiple linear regression analysis on pure and mixed solvent systems to reveal the solute–solvent and solvent–solvent interactions. Finally, the modified Apelblat model, the Jouyban–Acree model, and the Apelblat–Jouyban–Acree model were utilized to correlate the solubility data.
中文翻译:

d(-)-Salicin在纯和二元溶剂体系中的溶解度的测定和相关性
d(-)-水杨素在十二种纯溶剂(甲醇,乙醇,正丙醇,异丙醇,正丁醇,异丁醇,正戊醇,丙酮,乙腈,1,4-二恶英,乙酸乙酯和水)中的实验溶解度数据)和4种二元溶剂(水+乙醇,水+丙酮,水+异丙醇和水+甲醇)在大气压下测定的温度为283.15至323.15 K. 当溶剂组成恒定时,d的正相关在所有研究的纯溶剂和混合溶剂系统中均发现(-)-水杨素的溶解度与实验温度的关系。在给定温度下,随着水的摩尔分数增加,在二元溶剂系统的溶解度曲线中发现拐点,这可能归因于共溶现象。对于单溶剂,d(-)-salicin的溶解度可能受某些因素的影响,例如极性,空间效应,偶极-极化性,氢键等。KAT-LSER模型用于对纯和混合溶剂系统进行多元线性回归分析,以揭示溶质-溶剂和溶剂-溶剂的相互作用。最后,修改后的Apelblat模型,Jouyban-Acree模型和Apelblat-Jouyban-Acree模型用于关联溶解度数据。
更新日期:2020-09-10
中文翻译:

d(-)-Salicin在纯和二元溶剂体系中的溶解度的测定和相关性
d(-)-水杨素在十二种纯溶剂(甲醇,乙醇,正丙醇,异丙醇,正丁醇,异丁醇,正戊醇,丙酮,乙腈,1,4-二恶英,乙酸乙酯和水)中的实验溶解度数据)和4种二元溶剂(水+乙醇,水+丙酮,水+异丙醇和水+甲醇)在大气压下测定的温度为283.15至323.15 K. 当溶剂组成恒定时,d的正相关在所有研究的纯溶剂和混合溶剂系统中均发现(-)-水杨素的溶解度与实验温度的关系。在给定温度下,随着水的摩尔分数增加,在二元溶剂系统的溶解度曲线中发现拐点,这可能归因于共溶现象。对于单溶剂,d(-)-salicin的溶解度可能受某些因素的影响,例如极性,空间效应,偶极-极化性,氢键等。KAT-LSER模型用于对纯和混合溶剂系统进行多元线性回归分析,以揭示溶质-溶剂和溶剂-溶剂的相互作用。最后,修改后的Apelblat模型,Jouyban-Acree模型和Apelblat-Jouyban-Acree模型用于关联溶解度数据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号