当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Affinity‐switchable biotin probes for the analysis of enzymes and small reactive molecules on microarray platform
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-08-07 , DOI: 10.1002/jccs.202000200 Chia‐Lin Wu, Chen‐Yo Fan, Chun‐Cheng Lin, Kui‐Thong Tan
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-08-07 , DOI: 10.1002/jccs.202000200 Chia‐Lin Wu, Chen‐Yo Fan, Chun‐Cheng Lin, Kui‐Thong Tan
|
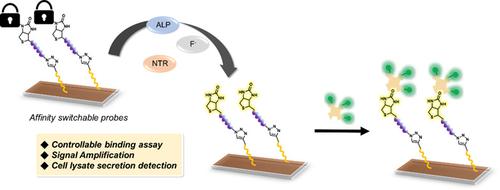
Array‐based analytical platforms have a distinct advantage of being able to detect analytes rapidly and simultaneously with the use of very small quantities of reagents and samples. However, the analyses of enzyme activities using this technique remain challenging, as the heterogeneous interface between the enzyme in the solution and the probe on the array surface impede the efficient binding and the subsequent enzymatic reaction. In this paper, we showed that the combination of affinity‐switchable biotin (ASB) probes and a novel click‐chemistry based immobilization method can overcome this heterogeneity problem. Three ASB probes were synthesized for the detection of alkaline phosphatase, nitroreductase, and the fluoride ion. This approach can be applied to determine the relative levels of phosphatase in different cell lines. We believe that the new strategy holds great potential for the effective sensing of a wide range of biomolecules in the future.
中文翻译:

亲和力可切换的生物素探针,用于在微阵列平台上分析酶和小的反应分子
基于阵列的分析平台具有显着的优势,即能够使用少量试剂和样品同时快速,同时检测分析物。然而,使用该技术进行酶活性分析仍然具有挑战性,因为溶液中酶与阵列表面探针之间的异质界面阻碍了有效结合和随后的酶促反应。在本文中,我们证明了将亲和力可转换生物素(ASB)探针与一种新颖的基于点击化学的固定化方法相结合可以克服这种异质性问题。合成了三种ASB探针,用于检测碱性磷酸酶,硝基还原酶和氟离子。该方法可用于确定不同细胞系中磷酸酶的相对水平。
更新日期:2020-08-07
中文翻译:

亲和力可切换的生物素探针,用于在微阵列平台上分析酶和小的反应分子
基于阵列的分析平台具有显着的优势,即能够使用少量试剂和样品同时快速,同时检测分析物。然而,使用该技术进行酶活性分析仍然具有挑战性,因为溶液中酶与阵列表面探针之间的异质界面阻碍了有效结合和随后的酶促反应。在本文中,我们证明了将亲和力可转换生物素(ASB)探针与一种新颖的基于点击化学的固定化方法相结合可以克服这种异质性问题。合成了三种ASB探针,用于检测碱性磷酸酶,硝基还原酶和氟离子。该方法可用于确定不同细胞系中磷酸酶的相对水平。



























 京公网安备 11010802027423号
京公网安备 11010802027423号