Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conformation modification of terthiophene during the on-surface synthesis of pure polythiophene.
Nanoscale ( IF 6.7 ) Pub Date : 2020-08-06 , DOI: 10.1039/d0nr04529b Liqian Liu 1 , Xinrui Miao 1 , Tingting Shi 2 , Xiaogang Liu 1 , Hin-Lap Yip 3 , Wenli Deng 1 , Yong Cao 1
Nanoscale ( IF 6.7 ) Pub Date : 2020-08-06 , DOI: 10.1039/d0nr04529b Liqian Liu 1 , Xinrui Miao 1 , Tingting Shi 2 , Xiaogang Liu 1 , Hin-Lap Yip 3 , Wenli Deng 1 , Yong Cao 1
Affiliation
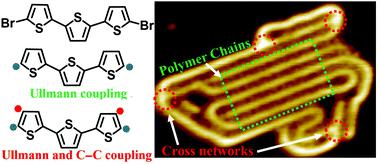
|
On-surface coupling under ultra-high vacuum is employed as a versatile approach to synthesize pure polythiophene from a 5,5′′-dibromo-2,2′:5′,2′′-terthiophene (DBTT) precursor and the corresponding temperature-dependent stepwise reaction mechanism is systematically studied by scanning tunneling microscopy (STM). After thermal deposition of the precursor onto a Au(111) surface that is kept at room temperature, a triangle-like pattern and a linear self-assembled pattern are formed with different molecular coverages through Br⋯Br⋯S halogen bonds and Br⋯Br type-I contact bonds, respectively. In the self-assembled nanostructures, the thiophene units adopt trans-conformation. Mild annealing promotes the structural transition of both nanostructures into ordered zigzag organometallic linear chains with all-cis configured thiophene units connected through coordination bonds to the Au adatoms. Such conformational variety is easily recognized by STM, particularly in the case of DBTT-CH3 with the extra –CH3 signals. The covalently coupled products from the DBTT precursor are obtained by further annealing the organometallic intermediate at higher temperatures, which leads to the removal of Au atoms and the formation of ordered polymer chains and disordered polythiophene networks. Further characterization suggests that the reaction mechanism is associated with Ullmann-type coupling to form the ordered chains as well as Ullmann-type and dehydrogenative C–C coupling to fabricate cross-linked polymer networks. Compared with the on-surface synthesis process of DBTT on the Cu(111) surface, it can be confirmed that the Au adatoms are vital to synthesize polythiophene. These findings provide important insight into the reaction mechanism of on-surface synthesized pure polythiophene and on-surface coupling can potentially be applied to synthesize other functional conjugated polymers.
中文翻译:

纯聚噻吩的表面合成过程中对噻吩的构象修饰。
超高真空下的表面偶联被用作从5,5'-二溴-2,2':5',2''-对噻吩(DBTT)前体和相应温度合成纯聚噻吩的通用方法扫描隧道显微镜(STM)系统研究了依赖于反应的逐步反应机理。将前体热沉积到保持在室温的Au(111)表面上后,通过Br⋯Br⋯S卤素键和Br⋯Br形成具有不同分子覆盖率的三角形图案和线性自组装图案I型接触键。在自组装的纳米结构中,噻吩单元采用反式构象。温和的退火促进了两个纳米结构的结构转变为有序的之字形有机金属线性链,顺式配置的噻吩单元通过配位键连接到Au原子上。这种构象变化很容易被STM识别,尤其是在带有额外–CH 3的DBTT-CH 3情况下信号。DBTT前体的共价偶联产物是通过在较高温度下进一步退火有机金属中间体而获得的,这导致去除Au原子并形成有序的聚合物链和无序的聚噻吩网络。进一步的特征表明,该反应机理与Ullmann型偶联形成有序链以及Ullmann型脱氢C–C偶联形成交联的聚合物网络有关。与DBTT在Cu(111)表面上的表面合成工艺相比,可以证实Au原子对于合成聚噻吩至关重要。
更新日期:2020-09-18
中文翻译:

纯聚噻吩的表面合成过程中对噻吩的构象修饰。
超高真空下的表面偶联被用作从5,5'-二溴-2,2':5',2''-对噻吩(DBTT)前体和相应温度合成纯聚噻吩的通用方法扫描隧道显微镜(STM)系统研究了依赖于反应的逐步反应机理。将前体热沉积到保持在室温的Au(111)表面上后,通过Br⋯Br⋯S卤素键和Br⋯Br形成具有不同分子覆盖率的三角形图案和线性自组装图案I型接触键。在自组装的纳米结构中,噻吩单元采用反式构象。温和的退火促进了两个纳米结构的结构转变为有序的之字形有机金属线性链,顺式配置的噻吩单元通过配位键连接到Au原子上。这种构象变化很容易被STM识别,尤其是在带有额外–CH 3的DBTT-CH 3情况下信号。DBTT前体的共价偶联产物是通过在较高温度下进一步退火有机金属中间体而获得的,这导致去除Au原子并形成有序的聚合物链和无序的聚噻吩网络。进一步的特征表明,该反应机理与Ullmann型偶联形成有序链以及Ullmann型脱氢C–C偶联形成交联的聚合物网络有关。与DBTT在Cu(111)表面上的表面合成工艺相比,可以证实Au原子对于合成聚噻吩至关重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号