当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
C2-Symmetric 1,2-Diphenylethane-1,2-diamine-Derived Primary-Tertiary Diamine-Catalyzed Asymmetric Mannich Addition of Cyclic N-Sulfonyl Trifluoromethylated Ketimines.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.joc.0c01446 Hui-Xin Duan 1 , Yongna Zhang 1 , Zhen-Zhen Zhang 1 , You-Qing Wang 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.joc.0c01446 Hui-Xin Duan 1 , Yongna Zhang 1 , Zhen-Zhen Zhang 1 , You-Qing Wang 1
Affiliation
|
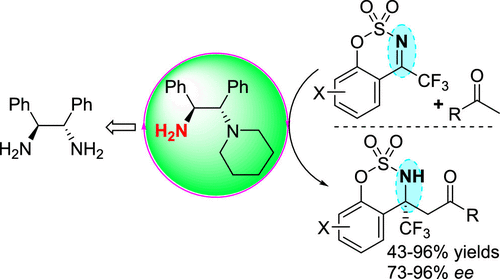
A simple chiral primary–tertiary diamine derived from C2-symmetric 1,2-diphenylethane-1,2-diamine as the organocatalyst in combination with the trifluoroacetic acid additive for the asymmetric Mannich reaction of cyclic N-sulfonyl trifluoromethylated ketimines and methyl ketones afforded the desired product with high enantioselectivity (73–96% ee). The reactions proceeded well for a variety of different substituted cyclic N-sulfonyl trifluoromethyl ketimines and various alkyl methyl ketones, providing access to diverse enantioenriched benzo-fused cyclic sulfamidate N-heterocycles bearing a trifluoromethylated α-tetrasubstituted carbon stereocenter. This study also investigated the diastereoselective reduction of the carbonyl group and ring cleavage reduction of the sulfamidate group of the corresponding Mannich product.
中文翻译:

C 2-对称的1,2-二苯乙烷-1,2-二胺衍生的伯叔叔二胺催化的环状N-磺酰基三氟甲基化的亚胺基亚胺的不对称曼尼希加成。
一种简单的手性伯-叔二胺,由C 2对称的1,2-二苯乙烷-1,2-二胺作为有机催化剂,与三氟乙酸添加剂结合使用,可进行环状N-磺酰基三氟甲基化酮亚胺和甲基酮的不对称曼尼希反应。具有高对映选择性(73–96%ee)的所需产品。对于各种不同的取代的环N-磺酰基三氟甲基酮亚胺和各种烷基甲基酮,反应进行得很好,从而提供了各种对映体富集的苯并稠合的环状氨基磺酸酯N。-带有三氟甲基化的α-四取代碳立体中心的杂环。这项研究还研究了相应曼尼希产品的羰基的非对映选择性还原和磺酰胺基的环裂解还原。
更新日期:2020-09-05
中文翻译:

C 2-对称的1,2-二苯乙烷-1,2-二胺衍生的伯叔叔二胺催化的环状N-磺酰基三氟甲基化的亚胺基亚胺的不对称曼尼希加成。
一种简单的手性伯-叔二胺,由C 2对称的1,2-二苯乙烷-1,2-二胺作为有机催化剂,与三氟乙酸添加剂结合使用,可进行环状N-磺酰基三氟甲基化酮亚胺和甲基酮的不对称曼尼希反应。具有高对映选择性(73–96%ee)的所需产品。对于各种不同的取代的环N-磺酰基三氟甲基酮亚胺和各种烷基甲基酮,反应进行得很好,从而提供了各种对映体富集的苯并稠合的环状氨基磺酸酯N。-带有三氟甲基化的α-四取代碳立体中心的杂环。这项研究还研究了相应曼尼希产品的羰基的非对映选择性还原和磺酰胺基的环裂解还原。

























 京公网安备 11010802027423号
京公网安备 11010802027423号