当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (+)-Raputindole A: An Iridium-Catalyzed Cyclization Approach.
Organic Letters ( IF 5.2 ) Pub Date : 2020-08-05 , DOI: 10.1021/acs.orglett.0c01943 Juliana L L F Regueira 1, 2 , Luiz F Silva 1 , Ronaldo A Pilli 2
Organic Letters ( IF 5.2 ) Pub Date : 2020-08-05 , DOI: 10.1021/acs.orglett.0c01943 Juliana L L F Regueira 1, 2 , Luiz F Silva 1 , Ronaldo A Pilli 2
Affiliation
|
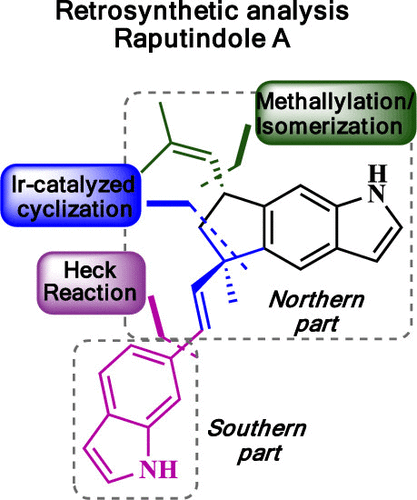
This work describes the total synthesis of raputindole A (1) through a convergent approach that features (1) an iridium-catalyzed cyclization to assemble the tricyclic core of the northern part, (2) enzymatic resolution to secure the preparation of an enantiomerically pure benzylic alcohol intermediate, and (3) the installation of the isobutenyl side chain via methallylation of the corresponding benzylic carbocation and coupling of the northern and southern parts via the Heck reaction. (+)-Raputindole A (1) was prepared in 10 steps (longest linear sequence) in 3.3% overall yield.
中文翻译:

(+)-Raputindole A的全合成:铱催化的环化方法。
这项工作描述了通过收敛方法的总合成Raputindole A(1),该方法的特征是(1)铱催化的环化反应组装北部的三环核,(2)酶促拆分,以确保对映体纯苄基的制备醇中间体,和(3)通过相应的苄基碳正离子的甲基化使异丁烯基侧链安装,并通过Heck反应使北部和南部部分偶联。(+)-Raputindole A(1)分10步(最长的线性序列)制备,总产率为3.3%。
更新日期:2020-08-21
中文翻译:

(+)-Raputindole A的全合成:铱催化的环化方法。
这项工作描述了通过收敛方法的总合成Raputindole A(1),该方法的特征是(1)铱催化的环化反应组装北部的三环核,(2)酶促拆分,以确保对映体纯苄基的制备醇中间体,和(3)通过相应的苄基碳正离子的甲基化使异丁烯基侧链安装,并通过Heck反应使北部和南部部分偶联。(+)-Raputindole A(1)分10步(最长的线性序列)制备,总产率为3.3%。


























 京公网安备 11010802027423号
京公网安备 11010802027423号