当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Densities and Viscosities of Binary Mixtures Containing Methyl Isobutyl Ketone and (C6–C10) 1-Alkanol
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.jced.0c00335 Mohammad Almasi 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.jced.0c00335 Mohammad Almasi 1
Affiliation
|
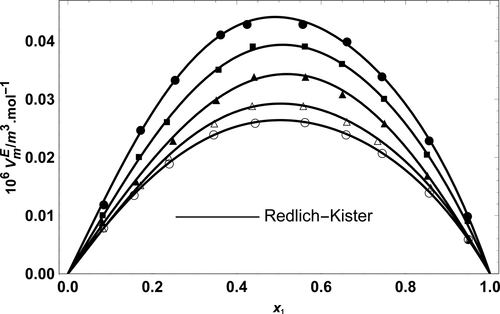
In the present study, to identify the governing intermolecular interactions in the binary mixtures of methyl isobutyl ketone (MIBK) and 1-alkanols (1-hexanol up to 1-decanol), experimental values of density and viscosity at temperatures (293.15 to 323.15 K) were reported. Excess molar volumes (VmE) and viscosity deviations (Δη) for mentioned binary mixtures were calculated and correlated by the Redlich–Kister polynomial expansions. It was found that increasing the alkyl chain of alcohol boosts the strength of intermolecular forces. Also, to obtain more information about the governing forces in the binary mixtures, values of Hansen solubility parameter distance between two molecules, Ra, were calculated, and based on the obtained results, the tendency of alcohol to dissolve in the MIBK media was discussed.
中文翻译:

含甲基异丁酮和(C 6 -C 10)1-烷醇的二元混合物的密度和粘度
在本研究中,要确定甲基异丁基酮(MIBK)和1-链烷醇(1-己醇直至1-癸醇)的二元混合物中支配性的分子间相互作用,在温度(293.15至323.15 K)下的密度和粘度实验值)的报告。计算出上述二元混合物的过量摩尔体积(V m E)和粘度偏差(Δη),并通过Redlich-Kister多项式展开式进行关联。发现增加醇的烷基链可增强分子间力的强度。同样,为了获得有关二元混合物中控制力的更多信息,两个分子之间的汉森溶解度参数距离值R a进行计算,并基于获得的结果,讨论了醇溶解在MIBK介质中的趋势。
更新日期:2020-09-10
中文翻译:

含甲基异丁酮和(C 6 -C 10)1-烷醇的二元混合物的密度和粘度
在本研究中,要确定甲基异丁基酮(MIBK)和1-链烷醇(1-己醇直至1-癸醇)的二元混合物中支配性的分子间相互作用,在温度(293.15至323.15 K)下的密度和粘度实验值)的报告。计算出上述二元混合物的过量摩尔体积(V m E)和粘度偏差(Δη),并通过Redlich-Kister多项式展开式进行关联。发现增加醇的烷基链可增强分子间力的强度。同样,为了获得有关二元混合物中控制力的更多信息,两个分子之间的汉森溶解度参数距离值R a进行计算,并基于获得的结果,讨论了醇溶解在MIBK介质中的趋势。


























 京公网安备 11010802027423号
京公网安备 11010802027423号