当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Proteolytic cleavage of Trop2 at Arg87 is mediated by matriptase and regulated by Val194
FEBS Letters ( IF 3.5 ) Pub Date : 2020-08-30 , DOI: 10.1002/1873-3468.13899 Pradnya R Kamble 1 , Sanjana Rane 1 , Ananya A Breed 1 , Shaini Joseph 2 , Smita D Mahale 1 , Bhakti R Pathak 1
FEBS Letters ( IF 3.5 ) Pub Date : 2020-08-30 , DOI: 10.1002/1873-3468.13899 Pradnya R Kamble 1 , Sanjana Rane 1 , Ananya A Breed 1 , Shaini Joseph 2 , Smita D Mahale 1 , Bhakti R Pathak 1
Affiliation
|
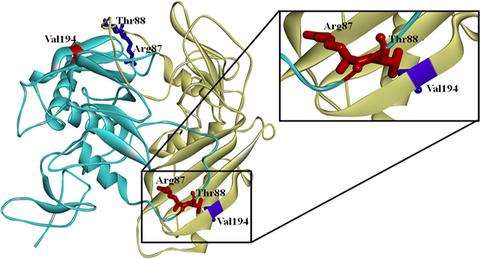
Proteolytic processing is an important post‐translational modification affecting protein activity and stability. In the current study, we investigate the N‐terminal cleavage of Trop2, a protein which is overexpressed in many cancers. We demonstrate that Trop2 is cleaved at Arg87 by a transmembrane serine protease, matriptase. Homology modeling and site‐directed mutagenesis of amino acids in close proximity to the matriptase cleavage site reveal the importance of Val194 in regulating Trop2 cleavage. Co‐immunoprecipitation studies confirm that amino acid substitutions at Arg87, Thr88, Lys189, Val194, and His195 do not affect Trop2 dimerization. However, cleavage of wild‐type Trop2 by matriptase is inhibited when it is allowed to dimerize with a V194A mutant monomer, further confirming the role of Val194 in matriptase‐mediated N‐terminal cleavage.
中文翻译:

Trop2 在 Arg87 的蛋白水解切割由 Matriptase 介导,并由 Val194 调节
蛋白水解加工是影响蛋白质活性和稳定性的重要翻译后修饰。在当前的研究中,我们研究了 Trop2 的 N 端切割,Trop2 是一种在许多癌症中过度表达的蛋白质。我们证明 Trop2 在 Arg87 处被跨膜丝氨酸蛋白酶、matriptase 切割。靠近matriptase切割位点的氨基酸的同源建模和定点诱变揭示了Val194在调节Trop2切割中的重要性。免疫共沉淀研究证实 Arg87、Thr88、Lys189、Val194 和 His195 处的氨基酸取代不影响 Trop2 二聚化。然而,当允许与 V194A 突变单体二聚化时,matriptase 对野生型 Trop2 的切割受到抑制,进一步证实了 Val194 在 Matriptase 介导的 N 端切割中的作用。
更新日期:2020-08-30
中文翻译:

Trop2 在 Arg87 的蛋白水解切割由 Matriptase 介导,并由 Val194 调节
蛋白水解加工是影响蛋白质活性和稳定性的重要翻译后修饰。在当前的研究中,我们研究了 Trop2 的 N 端切割,Trop2 是一种在许多癌症中过度表达的蛋白质。我们证明 Trop2 在 Arg87 处被跨膜丝氨酸蛋白酶、matriptase 切割。靠近matriptase切割位点的氨基酸的同源建模和定点诱变揭示了Val194在调节Trop2切割中的重要性。免疫共沉淀研究证实 Arg87、Thr88、Lys189、Val194 和 His195 处的氨基酸取代不影响 Trop2 二聚化。然而,当允许与 V194A 突变单体二聚化时,matriptase 对野生型 Trop2 的切割受到抑制,进一步证实了 Val194 在 Matriptase 介导的 N 端切割中的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号