当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interface Concentrated‐Confinement Suppressing Cathode Dissolution in Water‐in‐Salt Electrolyte
Advanced Energy Materials ( IF 27.8 ) Pub Date : 2020-08-06 , DOI: 10.1002/aenm.202000665 Jinming Yue 1, 2 , Liangdong Lin 1 , Liwei Jiang 1, 2 , Qiangqiang Zhang 1, 2 , Yuxin Tong 1, 2 , Liumin Suo 1, 2, 3 , Yong‐sheng Hu 1 , Hong Li 1 , Xuejie Huang 1 , Liquan Chen 1
Advanced Energy Materials ( IF 27.8 ) Pub Date : 2020-08-06 , DOI: 10.1002/aenm.202000665 Jinming Yue 1, 2 , Liangdong Lin 1 , Liwei Jiang 1, 2 , Qiangqiang Zhang 1, 2 , Yuxin Tong 1, 2 , Liumin Suo 1, 2, 3 , Yong‐sheng Hu 1 , Hong Li 1 , Xuejie Huang 1 , Liquan Chen 1
Affiliation
|
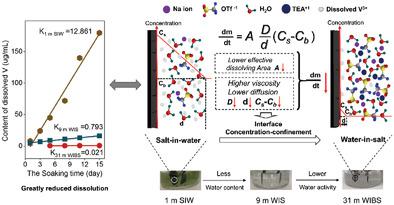
Mass dissolution is one main problems for cathodes in aqueous electrolytes due to the strong polarity of water molecules. In principle, mass dissolution is a thermodynamically favorable process as determined by the Gibbs free energy. However, in real situations, dissolution kinetics, which include viscosity, dissolving mass mobility, and interface properties, are also a critical factor influencing the dissolution rate. Both thermodynamic and kinetic dissolving factors can be regulated by the ratio of salt to solvent in the electrolyte. In this study, concentration‐controlled cathode dissolution is investigated in a susceptible Na3V2(PO4)3 cathode whose time‐, cycle‐, and state‐of‐charge‐dependent dissolubility are evaluated by multiple electrochemical and chemical methods. It is verified that the super‐highly concentrated water‐in‐salt electrolyte has a high viscosity, low vanadium ion diffusion, low polarity of solvated water, and scarce solute−water dissolving surfaces. These factors significantly lower the thermodynamic‐controlled solubility and the dissolving kinetics via time and physical space local mass interfacial confinement, thereby inducing a new mechanism of interface concentrated‐confinement which improves the cycling stability in real aqueous rechargeable sodium‐ion batteries.
中文翻译:

界面集中浓缩抑制盐溶水电解质中的阴极溶解
由于水分子的强极性,质量溶解是水性电解质中阴极的主要问题之一。原则上,质量溶解是由吉布斯自由能确定的热力学上有利的过程。但是,在实际情况下,包括粘度,溶解质量迁移率和界面特性在内的溶解动力学也是影响溶解速率的关键因素。热力学和动力学溶解因子均可通过电解质中盐与溶剂的比例来调节。在这项研究中,研究了在敏感的Na 3 V 2(PO 4)3中浓度受控的阴极溶解情况。通过多种电化学和化学方法评估阴极的时间,循环和荷电状态相关的溶解性。事实证明,超高浓度的盐包水电解质具有高粘度,低钒离子扩散,低极性的溶剂化水和稀疏的溶质水溶解表面。这些因素显着降低了热力学控制的溶解度,并通过时间和物理空间局部质量界面限制而降低了溶解动力学,从而引发了一种新的界面浓缩限制机制,从而提高了真正的水性可充电钠离子电池的循环稳定性。
更新日期:2020-09-22
中文翻译:

界面集中浓缩抑制盐溶水电解质中的阴极溶解
由于水分子的强极性,质量溶解是水性电解质中阴极的主要问题之一。原则上,质量溶解是由吉布斯自由能确定的热力学上有利的过程。但是,在实际情况下,包括粘度,溶解质量迁移率和界面特性在内的溶解动力学也是影响溶解速率的关键因素。热力学和动力学溶解因子均可通过电解质中盐与溶剂的比例来调节。在这项研究中,研究了在敏感的Na 3 V 2(PO 4)3中浓度受控的阴极溶解情况。通过多种电化学和化学方法评估阴极的时间,循环和荷电状态相关的溶解性。事实证明,超高浓度的盐包水电解质具有高粘度,低钒离子扩散,低极性的溶剂化水和稀疏的溶质水溶解表面。这些因素显着降低了热力学控制的溶解度,并通过时间和物理空间局部质量界面限制而降低了溶解动力学,从而引发了一种新的界面浓缩限制机制,从而提高了真正的水性可充电钠离子电池的循环稳定性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号