当前位置:
X-MOL 学术
›
J. Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efforts toward the synthesis of (+)-Lyconadin A
Journal of Chemical Sciences ( IF 1.7 ) Pub Date : 2020-08-06 , DOI: 10.1007/s12039-020-01771-8 SATISH KARELLA , SADAGOPAN RAGHAVAN
中文翻译:

合成(+)-Lyconadin A的努力

Journal of Chemical Sciences ( IF 1.7 ) Pub Date : 2020-08-06 , DOI: 10.1007/s12039-020-01771-8 SATISH KARELLA , SADAGOPAN RAGHAVAN
Abstract
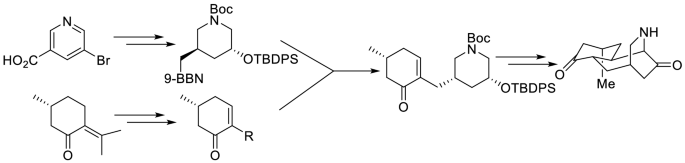
Synthetic efforts toward the synthesis of (+)-lyconadin A are described. B-Alkyl Suzuki coupling is utilized for combining 2-iodo cyclohexenone with the piperidine subunit. The piperidine subunit is derived from 5-bromo-3-nicotinic acid, and iodo cyclohexenone from commercially available (R)-pulegone. An intramolecular Michael reaction is employed for the creation of the C6-C7 bond.Graphic Abstract
Synthetic efforts toward the synthesis of (+)-lyconadin A are described. B-Alkyl Suzuki coupling is utilized to combine 2-iodo cyclohexenone with the piperidine subunit. The piperidine subunit is derived from 5-bromo-3-nicotinic acid, and iodo cyclohexenone from commercially available (R)-pulegone. An intramolecular Michael reaction is employed for creation of the C6-C7 bond.
中文翻译:

合成(+)-Lyconadin A的努力
摘要
描述了合成(+)-lyconadin A的合成方法。B-烷基Suzuki偶联用于将2-碘代环己烯酮与哌啶亚基结合。哌啶亚基衍生自5-溴-3-烟酸,碘代环己烯酮衍生自可商购的(R)-普勒高酮。分子内迈克尔反应用于产生C6-C7键。图形摘要
描述了合成(+)-lyconadin A的合成方法。B-烷基Suzuki偶联用于将2-碘代环己烯酮与哌啶亚基结合。哌啶亚基衍生自5-溴-3-烟酸,碘代环己烯酮衍生自可商购的(R)-普勒高酮。分子内迈克尔反应用于产生C6-C7键。


























 京公网安备 11010802027423号
京公网安备 11010802027423号