当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A comparative study on sulfide removal by HClO and KMnO4 in drinking water
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2020-08-05 , DOI: 10.1039/d0ew00629g Yeju Huang 1, 2, 3, 4 , Zaohong Liu 1, 2, 3, 4 , Yuanyuan Guo 1, 2, 3, 4 , Qin Lin 1, 2, 3, 4 , Xiaobin Liao 1, 2, 3, 4 , Huan Qi 4, 5, 6
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2020-08-05 , DOI: 10.1039/d0ew00629g Yeju Huang 1, 2, 3, 4 , Zaohong Liu 1, 2, 3, 4 , Yuanyuan Guo 1, 2, 3, 4 , Qin Lin 1, 2, 3, 4 , Xiaobin Liao 1, 2, 3, 4 , Huan Qi 4, 5, 6
Affiliation
|
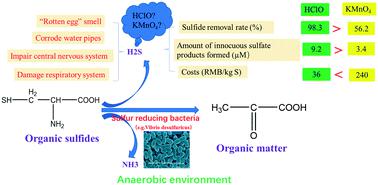
Sulfides, which are highly toxic to aquatic organisms and the human body, can be widely detected in water sources, especially in summer. Measures should be taken to eliminate them. In this paper, methods for the removal of sulfides and the formation of innocuous sulfate ion (SO42−) products using hypochlorite (HClO) and potassium permanganate (KMnO4) were compared; moreover, the effects of the oxidation conditions and co-existing components in the water were investigated. In addition, the proportions of different sulphur (S) species obtained using the two oxidants were evaluated. The results demonstrated that both oxidants are effective for sulfide removal, and the removal efficiency increased with increasing oxidant dosage. The removal rate of sulfides using HClO (97.8%) was higher than when using KMnO4 (87.6%) at the same oxidant dosage (3.2 mg L−1); moreover, more sulfides were transformed to innocuous SO42− when using the former (91.2%) than the latter (58.9%). The removal rate (from 97.4 to 30.9%) and proportion of SO42− formed (from 51.8 to 46.8%) both decreased upon raising the initial sulfide concentration from 0.2 to 0.8 mg L−1, while for KMnO4 these values decreased from 54.6 to 16.5%, and from 28.9 to 25.8%, respectively. Dissolved oxygen (DO) facilitated the removal of sulfides and the formation of SO42− when using either HClO or KMnO4. The presence of bicarbonate ions (HCO3−) raised the sulfide removal rate from 58.7 to 78.9% when using HClO, but it had little effect on removal using KMnO4. Co-existing SO42− facilitated the removal of sulfides during HClO oxidation but depressed their removal with KMnO4. Co-existing components in actual water matrices played minor roles in the sulfide removal process with HClO, but they had an inhibitory role when using KMnO4. These results can provide evidence for appropriately selecting a chemically effective method for oxidizing sulfides.
中文翻译:

饮用水中HClO和KMnO4去除硫化物的比较研究
对水生生物和人体有剧毒的硫化物可以在水源中广泛检测到,特别是在夏天。应该采取措施消除它们。本文使用次氯酸盐(HClO)和高锰酸钾(KMnO 4)去除硫化物和形成无害硫酸根离子(SO 4 2-)产物的方法)进行了比较;此外,研究了氧化条件和水中共存组分的影响。另外,评估了使用两种氧化剂获得的不同硫(S)物质的比例。结果表明,两种氧化剂对硫化物的去除均有效,去除效率随氧化剂用量的增加而增加。在相同的氧化剂剂量(3.2 mg L -1)下,使用HClO(97.8%)的硫化物去除率高于使用KMnO 4的(87.6%)。此外,使用前者(91.2%)比使用后者(58.9%)时,有更多的硫化物转化为无害的SO 4 2-。SO 4 2-的去除率(从97.4%降至30.9%)和比例当初始硫化物浓度从0.2 mg L -1提高时,生成的二氧化钛(从51.8降低到46.8%)都降低了,而对于KMnO 4,这些值分别从54.6降低到16.5%和从28.9降低到了25.8%。当使用HClO或KMnO 4时,溶解氧(DO)有助于硫化物的去除和SO 4 2-的形成。碳酸氢根离子的存在下(HCO 3 - ),使用的HClO当凸起从58.7硫化物去除率至78.9%,但对使用的KMnO去除效果少4。共存的SO 4 2-促进了HClO氧化过程中硫化物的去除,但抑制了其与KMnO 4的结合。实际水基质中的共存成分在用HClO去除硫化物的过程中起着较小的作用,但是当使用KMnO 4时它们具有抑制作用。这些结果可以适当地选择用于氧化硫化物化学有效的方法提供了证据。
更新日期:2020-10-02
中文翻译:

饮用水中HClO和KMnO4去除硫化物的比较研究
对水生生物和人体有剧毒的硫化物可以在水源中广泛检测到,特别是在夏天。应该采取措施消除它们。本文使用次氯酸盐(HClO)和高锰酸钾(KMnO 4)去除硫化物和形成无害硫酸根离子(SO 4 2-)产物的方法)进行了比较;此外,研究了氧化条件和水中共存组分的影响。另外,评估了使用两种氧化剂获得的不同硫(S)物质的比例。结果表明,两种氧化剂对硫化物的去除均有效,去除效率随氧化剂用量的增加而增加。在相同的氧化剂剂量(3.2 mg L -1)下,使用HClO(97.8%)的硫化物去除率高于使用KMnO 4的(87.6%)。此外,使用前者(91.2%)比使用后者(58.9%)时,有更多的硫化物转化为无害的SO 4 2-。SO 4 2-的去除率(从97.4%降至30.9%)和比例当初始硫化物浓度从0.2 mg L -1提高时,生成的二氧化钛(从51.8降低到46.8%)都降低了,而对于KMnO 4,这些值分别从54.6降低到16.5%和从28.9降低到了25.8%。当使用HClO或KMnO 4时,溶解氧(DO)有助于硫化物的去除和SO 4 2-的形成。碳酸氢根离子的存在下(HCO 3 - ),使用的HClO当凸起从58.7硫化物去除率至78.9%,但对使用的KMnO去除效果少4。共存的SO 4 2-促进了HClO氧化过程中硫化物的去除,但抑制了其与KMnO 4的结合。实际水基质中的共存成分在用HClO去除硫化物的过程中起着较小的作用,但是当使用KMnO 4时它们具有抑制作用。这些结果可以适当地选择用于氧化硫化物化学有效的方法提供了证据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号