Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2020-08-05 , DOI: 10.1016/j.jmgm.2020.107707 Ponciano García-Gutiérrez 1 , Rafael A Zubillaga 1 , Alexandro Téllez-Plancarte 1 , Roberto Flores-López 2 , Menandro Camarillo-Cadena 1 , Abraham Landa 2
|
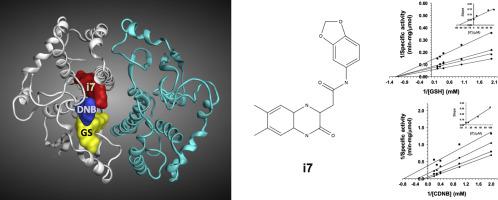
The inappropriate use of anthelmintics, such as praziquantel and albendazole, has generated resistance and the need to develop new drugs. Glutathione transferases, GSTs, are bisubstrate dimeric enzymes that constitute the main detoxification mechanism against electrophiles, drugs and oxidative damage in Taenia solium. Therefore, GSTs are important targets for the development of new anthelmintics. In this work, we reported a successful virtual screen aimed at the identification of novel inhibitors of a 26.5 kDa GST from T. solium (TsGST26). We found that a compound, i7, able to inhibit selectively TsGST26 concerning human GSTs, showing a non-competitive inhibition mechanism towards substrate glutathione with a Ki (GSH) of 55.7 μM and mixed inhibition towards the electrophilic substrate 1-chloro-2,4-dinitrobenzene with a Ki (CDNB) of 8.64 μM. These results are in agreement with those of docking simulations, which showed i7 binds a site adjacent to the electrophilic site and furthest from the glutathione site.
中文翻译:

通过虚拟筛选从Ta虫中发现了一种新的26.5 kDa谷胱甘肽转移酶的非底物抑制剂。
吡喹酮和阿苯达唑等驱虫药的不当使用产生了耐药性,并且需要开发新药。谷胱甘肽转移酶,是构成针对亲电子,药物和在氧化性损伤的主要解毒机制双底物二聚体酶猪带绦虫。因此,消费税是开发新型驱虫药的重要目标。在这项工作中,我们报告了成功的虚拟筛选,其目的是从T. solium(TsGST26)鉴定26.5 kDa GST的新型抑制剂。我们发现一种化合物i7能够选择性抑制有关人GST的TsGST26,显示出对具有K i的底物谷胱甘肽的非竞争性抑制机制(GSH)为55.7μM,并且对亲电底物1-氯-2,4-二硝基苯的混合抑制作用的K i(CDNB)为8.64μM。这些结果与对接模拟的结果相符,对接模拟显示i7结合了一个亲电位点且距离谷胱甘肽位点最远的位点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号