Comparative Biochemistry and Physiology B: Biochemistry & Molecular Biology ( IF 2.2 ) Pub Date : 2020-08-05 , DOI: 10.1016/j.cbpb.2020.110481 Anushka Vidurangi Samaraweera 1 , D S Liyanage 1 , W K M Omeka 1 , Hyerim Yang 1 , Thanthrige Thiunuwan Priyathilaka 2 , Jehee Lee 1
|
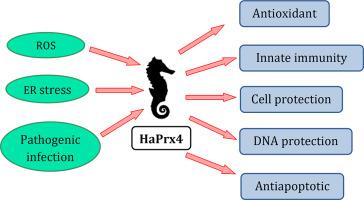
Peroxiredoxins (Prxs) are cysteine-dependent antioxidant proteins that play a leading part in oxidative stress response. Peroxiredoxin 4 (Prx4) is located in the endoplasmic reticulum, where it is primarily involved in regulating the concentration of H2O2, generated during protein folding. Prx4 is also located in the extracellular space, where it activates the JAK/STAT-mediated stress response. Here, we focus on the identification and characterization of the sequence and function of Prx4 from the big-belly seahorse (Hippocampus abdominalis) (HaPrx4). The size of the HaPrx4 coding sequence was 777 bp, which encoded a 258 amino acid protein of 28.8 kDa molecular weight. The amino acid sequence comprises a signal peptide, two active sites with peroxidatic cysteine and resolving cysteine, catalytic triad, and peroxiredoxin superfamily domain. According to the tissue distribution results, ovaries exhibited the highest HaPrx4 expression level within fourteen examined tissues. The HaPrx4 transcriptional responses to four immune stimulants (lipopolysaccharides, polyinosinic: polycytidylic acid, Edwardsiella tarda, and Streptococcus iniae) were evaluated in the blood and liver tissues. Additionally, the functions of recombinant HaPrx4 protein were evaluated by metal ion-catalyzed oxidation assay, peroxidase activity assay, insulin reduction assay, cell viability assay, and Hoechst staining. The assay results confirmed that the functions of HaPrx4 involved DNA protection, hydrogen peroxide (H2O2) elimination, oxidoreductase activity, enhancing cell survival, and cell protection. The results of the current study propose that HaPrx4 is effectively involved in H2O2 scavenging activity during stress conditions and in innate immune responses of the big-belly seahorse.
中文翻译:

来自大腹海马(腹部海马)的过氧化物酶毒素4(HaPrx4)的分子见解:分子特征,功能活性和针对免疫刺激剂的转录反应。
Peroxiredoxins(Prxs)是半胱氨酸依赖性的抗氧化剂蛋白,在氧化应激反应中起主要作用。Peroxiredoxin 4(Prx4)位于内质网,主要参与调节蛋白质折叠过程中产生的H 2 O 2浓度。Prx4也位于细胞外空间,在其中激活JAK / STAT介导的应激反应。在这里,我们着重于大腹海马(海马腹部) Prx4的序列和功能的鉴定和表征。)(HaPrx4)。HaPrx4编码序列的大小为777 bp,编码一个分子量为28.8 kDa的258个氨基酸的蛋白质。氨基酸序列包含信号肽,具有过氧化物半胱氨酸和分辨半胱氨酸的两个活性位点,催化三联体和过氧化物酶体超家族结构域。根据组织分布结果,卵巢在十四个受检组织中表现出最高的HaPrx4表达水平。该HaPrx4转录反应到四个免疫刺激(脂多糖,聚肌胞:胞苷酸,爱德华氏菌,和海豚链球菌)在血液和肝脏组织中进行了评估。另外,通过金属离子催化氧化测定,过氧化物酶活性测定,胰岛素减少测定,细胞生存力测定和Hoechst染色评价了重组HaPrx4蛋白的功能。分析结果证实,HaPrx4的功能涉及DNA保护,过氧化氢(H 2 O 2)消除,氧化还原酶活性,增强细胞存活和细胞保护。目前的研究结果表明,HaPrx4有效参与了应激条件下的H 2 O 2清除活性以及大腹海马的先天免疫反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号