当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dynamics and Mechanism of Binding of Androstenedione to Membrane-Associated Aromatase.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-03 , DOI: 10.1021/acs.biochem.0c00460 Lorela Paço 1 , Francisco Zarate-Perez 2 , Amanda F Clouser 1 , William M Atkins 1 , John C Hackett 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-03 , DOI: 10.1021/acs.biochem.0c00460 Lorela Paço 1 , Francisco Zarate-Perez 2 , Amanda F Clouser 1 , William M Atkins 1 , John C Hackett 2
Affiliation
|
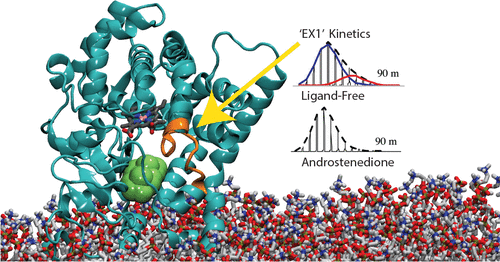
Aromatase (CYP19A1) catalyzes the synthesis of estrogens from androgens and is an invaluable target of pharmacotherapy for estrogen-dependent cancers. CYP19A1 is also one of the most primordial human CYPs and, to the extent that its fundamental dynamics are conserved, is highly relevant to understanding those of the more recently evolved and promiscuous enzymes. A complementary approach employing molecular dynamics simulations and hydrogen–deuterium exchange mass spectrometry (HDX-MS) was employed to interrogate the changes in CYP19A1 dynamics coupled to binding androstenedione (ASD). Gaussian-accelerated molecular dynamics and HDX-MS agree that ASD globally suppresses CYP19A1 dynamics. Bimodal HDX patterns of the B′–C loop potentially arising from at least two conformations are present in free 19A1 only, supporting the possibility that conformational selection is operative. Random-acceleration molecular dynamics and adaptive biasing force simulations illuminate ASD’s binding pathway, predicting ASD capture in the lipid headgroups and a pathway to the active site shielded from solvent. Intriguingly, the predicted access channel in 19A1 aligns well with the steroid binding sites of other human sterol-oxidizing CYPs.
中文翻译:

雄烯二酮与膜相关的芳香化酶结合的动力学和机理。
芳香酶(CYP19A1)催化雄激素合成雌激素,是药物治疗雌激素依赖性癌症的重要目标。CYP19A1也是最原始的人类CYP之一,并且在其基本动力学被保守的程度上,与理解那些最近进化和混杂的酶高度相关。采用分子动力学模拟和氢-氘交换质谱(HDX-MS)的补充方法,询问与结合雄烯二酮(ASD)偶联的CYP19A1动力学变化。高斯加速的分子动力学和HDX-MS一致认为ASD全局抑制CYP19A1动力学。B'–C环的双峰HDX模式可能至少由两个构象引起,仅在自由19A1中存在,支持构象选择有效的可能性。随机加速分子动力学和自适应偏压力模拟阐明了ASD的结合途径,预测了ASD在脂质头基团中的捕获以及通往被溶剂屏蔽的活性位点的途径。有趣的是,19A1中的预测访问通道与其他人类甾醇氧化CYP的类固醇结合位点吻合良好。
更新日期:2020-08-25
中文翻译:

雄烯二酮与膜相关的芳香化酶结合的动力学和机理。
芳香酶(CYP19A1)催化雄激素合成雌激素,是药物治疗雌激素依赖性癌症的重要目标。CYP19A1也是最原始的人类CYP之一,并且在其基本动力学被保守的程度上,与理解那些最近进化和混杂的酶高度相关。采用分子动力学模拟和氢-氘交换质谱(HDX-MS)的补充方法,询问与结合雄烯二酮(ASD)偶联的CYP19A1动力学变化。高斯加速的分子动力学和HDX-MS一致认为ASD全局抑制CYP19A1动力学。B'–C环的双峰HDX模式可能至少由两个构象引起,仅在自由19A1中存在,支持构象选择有效的可能性。随机加速分子动力学和自适应偏压力模拟阐明了ASD的结合途径,预测了ASD在脂质头基团中的捕获以及通往被溶剂屏蔽的活性位点的途径。有趣的是,19A1中的预测访问通道与其他人类甾醇氧化CYP的类固醇结合位点吻合良好。



























 京公网安备 11010802027423号
京公网安备 11010802027423号