当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel Tetrafunctional Probes Identify Target Receptors and Binding Sites of Small-Molecule Drugs from Living Systems.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-08-04 , DOI: 10.1021/acschembio.0c00335 Rin Miyajima 1 , Koji Sakai 1 , Yuki Otani 2 , Takashi Wadatsu 2 , Yasuyo Sakata 3 , Yuki Nishikawa 1 , Masaki Tanaka 2 , Yu Yamashita 1 , Mikayo Hayashi 1 , Kazumi Kondo 4 , Takashi Hayashi 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-08-04 , DOI: 10.1021/acschembio.0c00335 Rin Miyajima 1 , Koji Sakai 1 , Yuki Otani 2 , Takashi Wadatsu 2 , Yasuyo Sakata 3 , Yuki Nishikawa 1 , Masaki Tanaka 2 , Yu Yamashita 1 , Mikayo Hayashi 1 , Kazumi Kondo 4 , Takashi Hayashi 2
Affiliation
|
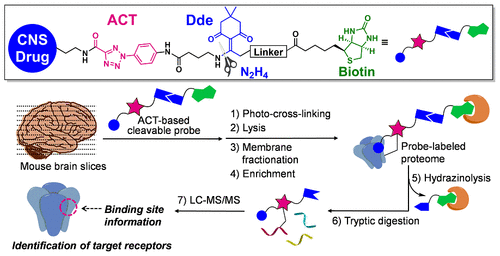
Significant advancement of chemoproteomics has contributed to uncovering the mechanism of action (MoA) of small-molecule drugs by characterizing drug–protein interactions in living systems. However, cell-membrane proteins such as G protein-coupled receptors (GPCRs) and ion channels, due to their low abundance and unique biophysical properties associated with multiple transmembrane domains, can present challenges for proteome-wide mapping of drug–receptor interactions. Herein, we describe the development of novel tetrafunctional probes, consisting of (1) a ligand of interest, (2) 2-aryl-5-carboxytetrazole (ACT) as a photoreactive group, (3) a hydrazine-labile cleavable linker, and (4) biotin for enrichment. In live cell labeling studies, we demonstrated that the ACT-based probe showed superior reactivity and selectivity for labeling on-target GPCR by mass spectrometry analysis compared with control probes including diazirine-based probes. By leveraging ACT-based cleavable probes, we further identified a set of representative ionotropic receptors, targeted by CNS drugs, with remarkable selectivity and precise binding site information from mouse brain slices. We anticipate that the robust chemoproteomic platform using the ACT-based cleavable probe coupled with phenotypic screening should promote identification of pharmacologically relevant target receptors of drug candidates and ultimately development of first-in-class drugs with novel MoA.
中文翻译:

新型四功能探针可识别来自生命系统的小分子药物的靶标受体和结合位点。
化学蛋白质组学的重大进步通过表征生物系统中药物与蛋白质的相互作用,为揭示小分子药物的作用机理(MoA)做出了贡献。但是,诸如G蛋白偶联受体(GPCR)和离子通道之类的细胞膜蛋白,由于其丰度低和与多个跨膜结构域相关的独特生物物理特性,可能对蛋白-药物相互作用的全蛋白组图谱提出挑战。在这里,我们描述了新型的四功能探针的开发,该探针由(1)感兴趣的配体,(2)作为光反应性基团的2-芳基-5-羧基四唑(ACT),(3)肼不稳定的可裂解连接子和(4)生物素的富集。在活细胞标记研究中,我们证明,与包括基于二嗪基的探针的对照探针相比,基于ACT的探针通过质谱分析显示出更高的反应活性和选择性,可用于标记目标GPCR。通过利用基于ACT的可裂解探针,我们进一步鉴定了一组具有代表性的离子受体,这些受体被CNS药物靶向,具有明显的选择性和来自小鼠脑片的精确结合位点信息。我们预计,使用基于ACT的可裂解探针结合表型筛选的强大的化学旋转平台应能促进候选药物的药理学相关靶标的鉴定,并最终开发出具有新型MoA的一流药物。我们进一步确定了一组具有代表性的离子性受体,它们以中枢神经系统药物为靶标,具有显着的选择性和来自小鼠脑片的精确结合位点信息。我们预计,使用基于ACT的可裂解探针结合表型筛选的强大的化学旋转平台应能促进候选药物的药理学相关靶标的鉴定,并最终开发出具有新型MoA的一流药物。我们进一步确定了一组具有代表性的离子性受体,它们以中枢神经系统药物为靶标,具有显着的选择性和来自小鼠脑片的精确结合位点信息。我们预计,使用基于ACT的可裂解探针结合表型筛选的强大的化学旋转平台应能促进候选药物的药理学相关靶标的鉴定,并最终开发出具有新型MoA的一流药物。
更新日期:2020-09-20
中文翻译:

新型四功能探针可识别来自生命系统的小分子药物的靶标受体和结合位点。
化学蛋白质组学的重大进步通过表征生物系统中药物与蛋白质的相互作用,为揭示小分子药物的作用机理(MoA)做出了贡献。但是,诸如G蛋白偶联受体(GPCR)和离子通道之类的细胞膜蛋白,由于其丰度低和与多个跨膜结构域相关的独特生物物理特性,可能对蛋白-药物相互作用的全蛋白组图谱提出挑战。在这里,我们描述了新型的四功能探针的开发,该探针由(1)感兴趣的配体,(2)作为光反应性基团的2-芳基-5-羧基四唑(ACT),(3)肼不稳定的可裂解连接子和(4)生物素的富集。在活细胞标记研究中,我们证明,与包括基于二嗪基的探针的对照探针相比,基于ACT的探针通过质谱分析显示出更高的反应活性和选择性,可用于标记目标GPCR。通过利用基于ACT的可裂解探针,我们进一步鉴定了一组具有代表性的离子受体,这些受体被CNS药物靶向,具有明显的选择性和来自小鼠脑片的精确结合位点信息。我们预计,使用基于ACT的可裂解探针结合表型筛选的强大的化学旋转平台应能促进候选药物的药理学相关靶标的鉴定,并最终开发出具有新型MoA的一流药物。我们进一步确定了一组具有代表性的离子性受体,它们以中枢神经系统药物为靶标,具有显着的选择性和来自小鼠脑片的精确结合位点信息。我们预计,使用基于ACT的可裂解探针结合表型筛选的强大的化学旋转平台应能促进候选药物的药理学相关靶标的鉴定,并最终开发出具有新型MoA的一流药物。我们进一步确定了一组具有代表性的离子性受体,它们以中枢神经系统药物为靶标,具有显着的选择性和来自小鼠脑片的精确结合位点信息。我们预计,使用基于ACT的可裂解探针结合表型筛选的强大的化学旋转平台应能促进候选药物的药理学相关靶标的鉴定,并最终开发出具有新型MoA的一流药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号