当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Towards analyzing the potential of exosomes to deliver microRNA therapeutics.
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-08-04 , DOI: 10.1002/jcp.29991 Lefki-Pavlina N Giassafaki 1 , Scheyla Siqueira 2 , Emmanuel Panteris 3 , Konstantina Psatha 4 , Fani Chatzopoulou 5 , Michalis Aivaliotis 4, 6, 7 , Georgios Tzimagiorgis 6, 7 , Anette Müllertz 2 , Dimitrios G Fatouros 8 , Ioannis S Vizirianakis 1, 7
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-08-04 , DOI: 10.1002/jcp.29991 Lefki-Pavlina N Giassafaki 1 , Scheyla Siqueira 2 , Emmanuel Panteris 3 , Konstantina Psatha 4 , Fani Chatzopoulou 5 , Michalis Aivaliotis 4, 6, 7 , Georgios Tzimagiorgis 6, 7 , Anette Müllertz 2 , Dimitrios G Fatouros 8 , Ioannis S Vizirianakis 1, 7
Affiliation
|
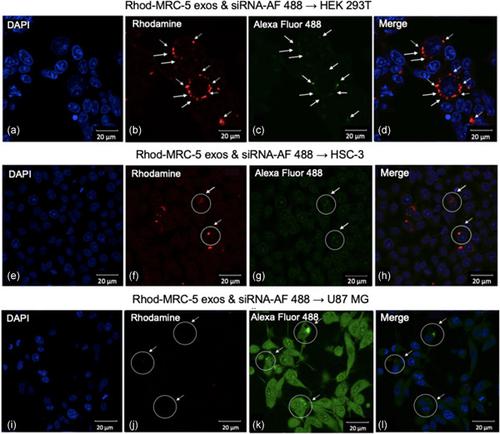
Exosome selectivity mechanisms underlying exosome–target cell interactions and the specific traits affecting their capability to communicate still remain unclear. Moreover, the capacity of exosomes to efficiently deliver their molecular cargos intracellularly needs precise investigation towards establishing functional exosome‐based delivery platforms exploitable in the clinical practice. The current study focuses on: (a) exosome production from normal MRC‐5 and Vero cells growing in culture, (b) physicochemical characterization by dynamic light scattering (DLS) and cryo‐transmission electron microscopy; (c) cellular uptake studies of rhodamine‐labeled exosomes in normal and cancer cells, providing to exosomes either “autologous” or “heterologous” cellular delivery environments; and (d) loading exogenous Alexa Fluor 488‐labeled siRNA into exosomes for the assessment of their delivering capacity by immunofluorescence in a panel of recipient cells. The data obtained thus far indicate that MRC‐5 and Vero exosomes, indeed exhibit an interesting delivering profile, as promising “bio‐shuttles,” being pharmacologically exploitable in the context of theranostic applications.
中文翻译:

分析外泌体提供 microRNA 疗法的潜力。
外泌体-靶细胞相互作用的外泌体选择性机制以及影响其交流能力的特定特征仍不清楚。此外,外泌体在细胞内有效递送其分子货物的能力需要精确研究,以建立可在临床实践中利用的基于功能性外泌体的递送平台。目前的研究重点是:(a)培养中生长的正常 MRC-5 和 Vero 细胞的外泌体产生,(b)通过动态光散射(DLS)和低温透射电子显微镜进行的物理化学表征;(c) 罗丹明标记外泌体在正常细胞和癌细胞中的细胞摄取研究,为外泌体提供“自体”或“异源”细胞递送环境;(d) 将外源性 Alexa Fluor 488 标记的 siRNA 加载到外泌体中,通过免疫荧光评估它们在一组受体细胞中的递送能力。迄今为止获得的数据表明,MRC-5 和 Vero 外泌体确实表现出一种有趣的传递特性,作为有希望的“生物穿梭”,在治疗诊断应用的背景下可在药理学上加以利用。
更新日期:2020-08-04
中文翻译:

分析外泌体提供 microRNA 疗法的潜力。
外泌体-靶细胞相互作用的外泌体选择性机制以及影响其交流能力的特定特征仍不清楚。此外,外泌体在细胞内有效递送其分子货物的能力需要精确研究,以建立可在临床实践中利用的基于功能性外泌体的递送平台。目前的研究重点是:(a)培养中生长的正常 MRC-5 和 Vero 细胞的外泌体产生,(b)通过动态光散射(DLS)和低温透射电子显微镜进行的物理化学表征;(c) 罗丹明标记外泌体在正常细胞和癌细胞中的细胞摄取研究,为外泌体提供“自体”或“异源”细胞递送环境;(d) 将外源性 Alexa Fluor 488 标记的 siRNA 加载到外泌体中,通过免疫荧光评估它们在一组受体细胞中的递送能力。迄今为止获得的数据表明,MRC-5 和 Vero 外泌体确实表现出一种有趣的传递特性,作为有希望的“生物穿梭”,在治疗诊断应用的背景下可在药理学上加以利用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号