当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Route to Indoles via Modified Fischer Indole Intermediates from Sulfonanilides and Ketene Dithioacetal Monoxides
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-08-04 , DOI: 10.1002/ajoc.202000397 Jun Kinoshita 1 , Alexandre Baralle 1 , Akira Yoshida 1 , Tomoyuki Yanagi 1 , Keisuke Nogi 1 , Hideki Yorimitsu 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-08-04 , DOI: 10.1002/ajoc.202000397 Jun Kinoshita 1 , Alexandre Baralle 1 , Akira Yoshida 1 , Tomoyuki Yanagi 1 , Keisuke Nogi 1 , Hideki Yorimitsu 1
Affiliation
|
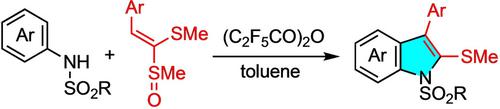
An S−N variant of the N−N‐based Fischer indole synthesis has been developed. Treatment of sulfonanilides and ketene dithioacetal monoxides with a powerful acid anhydride provides N‐sulfonyl‐2‐methylsulfanylindoles. The initial interrupted Pummerer reaction would yield the key S−N‐tethered precursor in situ that then undergoes [3,3] sigmatropic rearrangement, after which the endgame to the indole ring follows the Fischer manner.
中文翻译:

从磺酰苯胺和丁烯二硫缩醛一氧化物经修饰的费歇尔吲哚中间体到吲哚的途径
已开发出基于N-N的Fischer吲哚合成的S-N变体。用强力酸酐处理磺酰苯胺和烯酮二硫缩醛一氧化氮,可提供N-磺酰基-2-甲基磺胺基吲哚酯。最初中断的Pummerer反应将原位产生关键的SN系链前体,然后进行[3,3]σ重排,此后,吲哚环的终态遵循Fischer方式。
更新日期:2020-10-11
中文翻译:

从磺酰苯胺和丁烯二硫缩醛一氧化物经修饰的费歇尔吲哚中间体到吲哚的途径
已开发出基于N-N的Fischer吲哚合成的S-N变体。用强力酸酐处理磺酰苯胺和烯酮二硫缩醛一氧化氮,可提供N-磺酰基-2-甲基磺胺基吲哚酯。最初中断的Pummerer反应将原位产生关键的SN系链前体,然后进行[3,3]σ重排,此后,吲哚环的终态遵循Fischer方式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号