当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
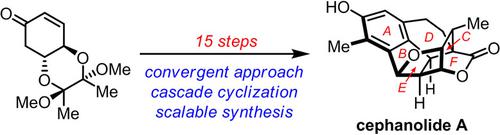
Asymmetric Total Synthesis of Cephanolide A.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-08-04 , DOI: 10.1002/anie.202009562 Hongyuan Zhang 1 , Haibing He 2 , Shuanhu Gao 1, 2
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-08-04 , DOI: 10.1002/anie.202009562 Hongyuan Zhang 1 , Haibing He 2 , Shuanhu Gao 1, 2
Affiliation
|
The first asymmetric total synthesis of cephanolide A, a complex hexacyclic C18 dinorditerpenoid from cephalotaxus sinensis, was achieved. The synthesis features a convergent strategy, which provides a flexible approach to prepare the biogenetically cephalotaxus diterpenoids and structurally related derivatives for biological studies. A mild intramolecular Prins cyclization was developed to construct the central hexahydrofluorenol skeleton (A‐B‐C ring), which relies on the originally proposed hydroacylation strategy. A remote hydroxy group directed hydrogenation was applied to stereospecifically reduce the tetra‐substituted enone unit. A sequence of ring forming steps, including lactonization, cation mediated etherification and Friedel–Crafts cyclization, was efficiently utilized to forge the cage‐like skeleton.
更新日期:2020-08-04


























 京公网安备 11010802027423号
京公网安备 11010802027423号