Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2020-08-04 , DOI: 10.1016/j.jmgm.2020.107703 Hassan Monhemi 1 , Seyedeh Samaneh Tabaee 2
|
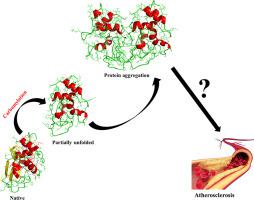
Amino acid mutations in some proteins such as lysozyme lead to genetically disorder variants and adverse pathogenic consequences. Recently, amino acid modifications were known as a risk factor in many related diseases such as uremia and atherosclerosis, showing the importance of these surface-structure changes. Although the structural consequences of the hereditary proteins have been examined extensively, such effects for the protein modifications are known to a lesser extent. One drawback in the examination of protein modifications is hardness in experimental detection of modifications by techniques such as NMR and crystallography. Molecular modeling and simulation can help to understand such phenomena at the molecular levels. It is more rational that the effects of both mutation and modification can be compared in a single protein model. Here, molecular dynamics simulation is used to compare the effects of a disease-related carbamylation modification and an amyloidogenic mutation (D67H) in human lysozyme as a model protein. The results show that the carbamylation adversely effects on the tertiary structure, leading to the similar unfolding pathway to the hereditary amyloidogenic form. The carbamylation leads to the instability of the overall protein conformation, especially on the β-domain, which is a characteristic of hereditary amyloidosis in human lysozymes. The aggregation behaviors of both modified and mutant lysozyme were examined by molecular docking calculations. The results showed that the partially unfolded lysozyme might form tight protein aggregates upon carbamylation similar to the amyloidogenic variant. Both single and all-residues carbamylations impose serious conformational changes to the tertiary structure of lysozyme. It was obtained that carbamylation of lysozyme strongly effects on the stability of N-terminal β-sheet, which can produce a highly unstable conformation. The results of this study not only show the adverse structural consequences of a disease-associated post-translational modification, but it also may be very helpful to understand the molecular basis for many carbamylation-related diseases such as atherosclerosis in ESRD patients. The results show that non-native post-translational modifications may be as structurally important as hereditary mutations.
中文翻译:

突变和修饰对人类溶菌酶的结构和稳定性的影响:氨基甲酰化与动脉粥样硬化之间的分子联系。
某些蛋白质(如溶菌酶)中的氨基酸突变会导致遗传疾病变异和不利的致病后果。最近,氨基酸修饰被认为是许多相关疾病(如尿毒症和动脉粥样硬化)的危险因素,显示出这些表面结构变化的重要性。尽管已经广泛地检查了遗传蛋白的结构后果,但是对于蛋白修饰的这种作用知之甚少。蛋白质修饰检查的一个缺点是通过诸如NMR和晶体学等技术对修饰进行实验检测的硬度。分子建模和模拟可以帮助从分子水平理解此类现象。可以在单个蛋白质模型中比较突变和修饰的影响是更合理的。这里,分子动力学模拟用于比较疾病相关的氨基甲酸酯化修饰和人类溶菌酶作为模型蛋白的淀粉样变性(D67H)的影响。结果表明,氨基甲酸酯化对三级结构有不利影响,导致类似的向遗传性淀粉样生成形式的展开途径。氨基甲酸酯化导致整体蛋白质构象的不稳定,尤其是在β结构域上,这是人类溶菌酶遗传性淀粉样变性的特征。通过分子对接计算检查了修饰的和突变的溶菌酶的聚集行为。结果表明,部分解折叠的溶菌酶可能在氨基甲酸酯化后形成紧密的蛋白质聚集体,类似于淀粉样蛋白生成变体。单残基和所有残基的氨甲酰化都对溶菌酶的三级结构施加了严重的构象变化。获得了溶菌酶的氨基甲酰化强烈影响N-末端β-折叠的稳定性,其可以产生高度不稳定的构象。这项研究的结果不仅显示了疾病相关的翻译后修饰的不利结构后果,而且对于了解许多与氨甲酰化相关的疾病(如ESRD患者的动脉粥样硬化)的分子基础也可能非常有帮助。结果表明,非天然翻译后修饰在结构上可能与遗传突变一样重要。会产生高度不稳定的构象。这项研究的结果不仅显示了疾病相关的翻译后修饰的不利结构后果,而且对于了解许多与氨甲酰化相关的疾病(如ESRD患者的动脉粥样硬化)的分子基础也可能非常有帮助。结果表明,非天然翻译后修饰在结构上可能与遗传突变一样重要。会产生高度不稳定的构象。这项研究的结果不仅显示了疾病相关的翻译后修饰的不利结构后果,而且对于了解许多与氨甲酰化相关的疾病(如ESRD患者的动脉粥样硬化)的分子基础也可能非常有帮助。结果表明,非天然翻译后修饰在结构上可能与遗传突变一样重要。

























 京公网安备 11010802027423号
京公网安备 11010802027423号