Journal of Catalysis ( IF 7.3 ) Pub Date : 2020-08-03 , DOI: 10.1016/j.jcat.2020.07.028 Garrett M. Mitchell , Kaiwalya D. Sabnis , Fred G. Sollberger , Yanran Cui , Chang Wan Han , Paulami Majumdar , Zhenhua Zeng , Jeffrey T. Miller , Jeffrey Greeley , Volkan Ortalan , Chao Wang , W. Nicholas Delgass , Fabio H. Ribeiro
|
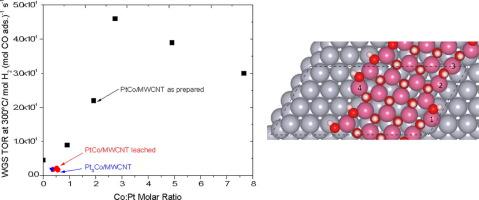
A series of cobalt-promoted Pt catalysts supported on multi-walled carbon nanotubes was synthesized, and their performance was evaluated for the water-gas shift (WGS) reaction. Compared to the monometallic Pt catalyst, the WGS turnover rate (TOR) at 300 °C was promoted by a factor of 10 at a Pt:Co molar ratio of 1:3. X-ray absorption spectroscopy and XRD showed the presence of a Pt3Co alloy along with a partially oxidized cobalt and a free cobalt metal phase after reduction pretreatment. In order to determine the dominant active site over the Co-promoted catalysts, selective leaching of partially oxidized Co (designated as CoOxHy) and Co metal was performed with a 5% wt. acetic acid solution, while preserving the Pt-rich phases. The WGS TOR at 300 °C for the Co-promoted catalysts after leaching was observed to be even lower than that of the monometallic Pt catalyst. Thus, the alloy formation was determined to be inconsequential towards promotion in the WGS TOR, while the dominant active site was determined to be a PtCo alloy in intimate contact with the CoOxHy phase. Combined Density Functional Theory (DFT) calculations and ab-initio thermodynamic phase diagrams point to a monolayer of CoOH being the most stable Co phase on Pt(1 1 1) under WGS conditions. Calculations of OH binding energies on Pt(1 1 1), Pt3Co(1 1 1), and at the interface between CoOH overlayers and Pt(1 1 1) show that trends in the WGS activity of these catalysts are linked to the strength of OH binding, with the strongest OH binding found at the interface between CoOH and Pt, supporting the conclusion that a similar interface is the source of enhanced WGS activity in the PtCo bimetallic system.
中文翻译:

钴对多壁碳纳米管负载水煤气变换铂的影响
合成了一系列负载在多壁碳纳米管上的钴促进的Pt催化剂,并对其水煤气变换(WGS)反应进行了性能评估。与单金属Pt催化剂相比,在Pt:Co摩尔比为1:3的情况下,在300°C下WGS的转化率(TOR)提高了10倍。X射线吸收光谱法和XRD显示还原预处理后存在Pt 3 Co合金以及部分氧化的钴和游离的钴金属相。为了确定共助催化剂上的主要活性位点,对部分氧化的钴(称为CoO x H y)进行选择性浸出),并以5%wt。乙酸溶液,同时保留富Pt相。共浸催化剂在浸出后在300°C时的WGS TOR甚至低于单金属Pt催化剂。因此,确定合金的形成对提升WGS TOR无关紧要,而确定的主要活性位点是与CoO x H y相紧密接触的PtCo合金。结合密度泛函理论(DFT)计算和从头算热力学相图,得出在WGS条件下,单层CoOH是Pt(1 1 1)上最稳定的Co相。Pt(1 1 1),Pt 3上的OH结合能的计算Co(1 1 1)以及CoOH覆盖层和Pt(1 1 1)之间的界面表明,这些催化剂的WGS活性趋势与OH结合强度有关,其中最强的OH结合CoOH和Pt,支持以下结论:类似的界面是PtCo双金属系统中WGS活性增强的来源。



























 京公网安备 11010802027423号
京公网安备 11010802027423号