Catalysis Communications ( IF 3.7 ) Pub Date : 2020-08-03 , DOI: 10.1016/j.catcom.2020.106128 Zsófia Császár , Eszter Z. Szabó , Attila C. Bényei , József Bakos , Gergely Farkas
|
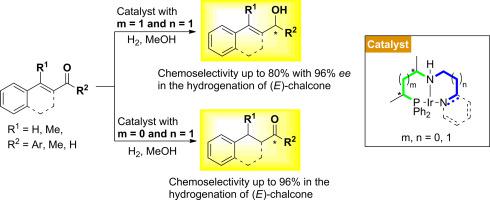
A novel, highly modular approach has been developed for the synthesis of new chiral P,N,N ligands with the general formula Ph2P(CH3)CH(CH2)mCH(CH3)NHCH2CH2(CH2)nN(CH3)2 and Ph2P(CH3)CHCH2CH(CH3)NHCH2(CH2)n-2-Py (m, n = 0, 1). The systematic variation of their PN and N
N backbone led to the conclusion that the activity, chemo- and enantioselectivity in the hydrogenation of α,β-unsaturated ketones are highly dependent on the combination of the two bridge lengths. It has been found that a minor change in the ligand's structure, i. e. varying the value of m from 1 to 0, can switch the chemoselectivity of the reaction, from 80% C
O to 97% C
C selectivity.
中文翻译:

Ir(P,N,N)配合物的螯合物环大小效应:α,β-不饱和酮不对称氢化中的化学选择性转换
已开发出一种新颖的,高度模块化的方法,用于合成通式为Ph 2 P(CH 3)CH(CH 2)m CH(CH 3)NHCH 2 CH 2(CH 2)的新手性P,N,N配体)n N(CH 3)2和Ph 2 P(CH 3)CHCH 2 CH(CH 3)NHCH 2(CH 2)n -2-Py(m,n = 0,1)。它们的PN和N的系统变化
N主链得出的结论是,α,β-不饱和酮氢化中的活性,化学选择性和对映选择性高度依赖于两个桥长度的组合。已经发现,配体结构的微小变化,即将m的值从1改变为0,可以将反应的化学选择性从80%
CO转化为97%
CC。


























 京公网安备 11010802027423号
京公网安备 11010802027423号