当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Applications of β‐Aminoalkylboronic Acid Derivatives
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-08-02 , DOI: 10.1002/adsc.202000690 Xiangyu Li 1 , Dennis G. Hall 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-08-02 , DOI: 10.1002/adsc.202000690 Xiangyu Li 1 , Dennis G. Hall 1
Affiliation
|
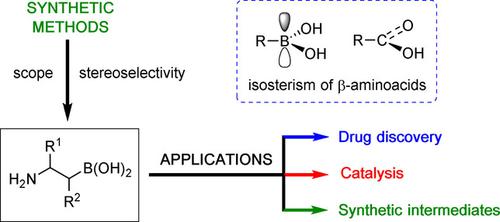
α‐Aminoalkylboronic acids display a distinct role in medicinal chemistry, and their utility has been demonstrated by the successful commercialization of three drugs: bortezomib, ixazomib, and vaborbactam. Just as α‐aminoalkylboronic acids are a bioisostere of α‐amino acids, β‐aminoalkylboronates are a bona fide bioisostere of β‐amino acids, thus they also hold promising potential in drug discovery. Moreover, β‐aminoalkylboronates are versatile synthetic intermediates that are amenable to many of the established C−B bond derivatization reactions of chiral optically enriched alkylboronates, leading to the stereocontrolled preparation of valued classes of products such as β‐amino alcohols, 1,2‐diamines, and hemiboronic acid heterocycles. In addition, β‐aminoalkylboronates were shown to act as catalysts in certain organic reactions. This review presents an overview of the strengths and limitations of current preparative methods to access β‐aminoalkylboronic acid derivatives stereoselectively with various substitution patterns. Strategically, several disconnections can be exploited to establish both functional groups. Some of the key methods include the classical Matteson asymmetric homologation chemistry, transition metal‐catalyzed aminoboration of alkenes and formal hydroboration of enamine derivatives, nucleophilic additions of boryl‐substituted carbanions onto N‐functionalized imines, borylative ring openings of aziridines, and functionalization of alpha‐boryl aldehydes.
中文翻译:

β-氨基烷基硼酸衍生物的合成及应用
α-氨基烷基硼酸在药物化学中显示出独特的作用,其有效性已通过三种药物的成功商业化证明:硼替佐米,依沙米单抗和vaborbactam。正如α-氨基烷基硼酸是α-氨基酸的生物等排体一样,β-氨基烷基硼酸酯是β-氨基酸的真正的生物等排体,因此它们在药物开发中也具有广阔的发展潜力。此外,β-氨基烷基硼酸酯是通用的合成中间体,适用于手性光学富集的烷基硼酸酯的许多已建立的C-B键衍生化反应,从而可以立体控制制备有价值的产品类别,例如β-氨基醇,1,2-二胺和半硼酸杂环。此外,已证明β-氨基烷基硼酸酯在某些有机反应中起催化剂的作用。这篇综述概述了目前制备方法的优势和局限性,这些制备方法以各种取代方式立体选择性地进入β-氨基烷基硼酸衍生物。从策略上讲,可以利用几个断开连接来建立两个功能组。一些关键方法包括经典的Matteson不对称同源化学,烯烃的过渡金属催化的氨基硼化和烯胺衍生物的形式氢硼化,硼取代的碳负离子的亲核加成N-官能化的亚胺,氮丙啶的硼化环开口和α-硼基醛的官能化。
更新日期:2020-08-02
中文翻译:

β-氨基烷基硼酸衍生物的合成及应用
α-氨基烷基硼酸在药物化学中显示出独特的作用,其有效性已通过三种药物的成功商业化证明:硼替佐米,依沙米单抗和vaborbactam。正如α-氨基烷基硼酸是α-氨基酸的生物等排体一样,β-氨基烷基硼酸酯是β-氨基酸的真正的生物等排体,因此它们在药物开发中也具有广阔的发展潜力。此外,β-氨基烷基硼酸酯是通用的合成中间体,适用于手性光学富集的烷基硼酸酯的许多已建立的C-B键衍生化反应,从而可以立体控制制备有价值的产品类别,例如β-氨基醇,1,2-二胺和半硼酸杂环。此外,已证明β-氨基烷基硼酸酯在某些有机反应中起催化剂的作用。这篇综述概述了目前制备方法的优势和局限性,这些制备方法以各种取代方式立体选择性地进入β-氨基烷基硼酸衍生物。从策略上讲,可以利用几个断开连接来建立两个功能组。一些关键方法包括经典的Matteson不对称同源化学,烯烃的过渡金属催化的氨基硼化和烯胺衍生物的形式氢硼化,硼取代的碳负离子的亲核加成N-官能化的亚胺,氮丙啶的硼化环开口和α-硼基醛的官能化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号